Results demonstrating safety and efficacy in those with anemia in non-dialysis dependent chronic kidney disease and anemia in dialysis-dependent chronic kidney disease were presented at the 2024 American Society of Nephrology Kidney Week.

Results demonstrating safety and efficacy in those with anemia in non-dialysis dependent chronic kidney disease and anemia in dialysis-dependent chronic kidney disease were presented at the 2024 American Society of Nephrology Kidney Week.

Nearly 19,000 independent pharmacies continue to work across the country, with more than 200,000 employees and millions of patients.

Nationally, 7 million women live in maternal health deserts, defined as counties with limited or no available obstetric care.

Changing the way pharmacists and pharmacy staff think about themselves is key, although providers, payers, and patients must also change how they view the value of pharmacy services.
With COVID-19, influenza, respiratory syncytial virus (RSV), and other viruses now circulating, the time for pharmacists to get up to date on the latest vaccine recommendations is now.

Nathan Vanderford, PhD, MBA speaks about barriers to cancer care access experienced by patients in rural communities.

Sara Rogers, PharmD, discusses the formation and goals of the Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) initiative, which seeks to standardize pharmacogenomic practices and improve clinical decision-making by establishing consensus across all stakeholders.

Ryan Nelson, PharmD, discusses the STRIPE Annual Meeting and Consensus Workshop’s focus on unifying pharmacogenetic guidelines across major organizations, such as the FDA, European Medicines Agency, Clinical Pharmacogenetics Implementation Consortium, and National Comprehensive Cancer Network.

Stephanie White, PharmD, CSP, is presenting at the NCODA Fall Summit in Orlando, Florida.
These therapies are being investigated in earlier lines, with several new treatments in development.

Benjamin Brown of Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) discusses his own experience as a patient with cancer and how consensus around pharmacogenomic practices can improve the lives of patients.
Cabotegravir long-acting when used for HIV pre-exposure prophylaxis (PrEP) showed adherence, effectiveness, and improvements in quality of life by those who received the injection.

Ginger Blackmon, PharmD, will discuss enhancing patient cancer care through implementation of medically integrated oncology team and NCODA’s PQI resources.

Pharmacy Times will be at the NCODA Fall Summit in Orlando from October 23 to October 25, 2024.

The guidelines emphasize the importance of vaccination, risk assessment, and preparedness for immunocompromised patients who are traveling post-transplant.
The KEYNOTE-756 and CheckMate 7FL trials show this combination improves pathological complete response rates in this patient population.
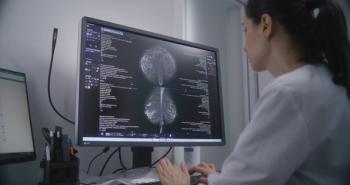
Pharmacogenomic testing can help identify patients' genetic profiles, revealing why certain drugs may be less effective or cause adverse effects.

Shirish Gadgeel, MD, discusses an integrated analysis of the regional TRUST-I study and global TRUST-II study presented at the European Society for Medical Oncology Congress 2024.

The FDA faces a multitude of challenges in regulating artificial intelligence (AI) and machine learning (ML) medical devices.

Terry Keys highlights the role of language and terminology as barriers for patients receiving cancer care.
Sarah Hudson-Disalle, PharmD, highlights the significant impact of EHR automation on biosimilar access and costs.

Nadine Barrett, PhD, MA, MPH, discusses the role of international collaborations to advance global cancer care access.

Naoto T. Ueno, MD, PhD, FACP, discussed the role of the Hawaiʻi Cancer Consortium to advance trial and treatment efforts for patients with cancer.

Courtney VanHouzen, PharmD shares insights about the community bispecific program at Munson Healthcare.

Shirish Gadgeel, MD, discusses the promising results of the randomized phase 3 HARMONi-2/AK112-303 study comparing ivonescimab to pembrolizumab in PD-L1–positive advanced non-small cell lung cancer.

Longer-term data from the phase 3 MARIPOSA trial confirm superior outcomes of a chemotherapy-free amivantamab-vmjw plus lazertinib regimen compared to osimertinib monotherapy as first-line therapy.

At the 2024 World Conference on Lung Cancer, Ana Baramidze, MD, PhD, presented 5-year outcomes of the trial, which investigated cemiplimab monotherapy in first-line advanced non–small cell lung cancer (NSCLC) with PD-L1 expression of 50% or greater.

Investigators report the study met its primary end point, demonstrating that a single, oral dose reduced the transmission by those infected to others in their household.

At the 2024 World Conference on Lung Cancer, Benjamin Besse, MD, PhD, discussed the findings of the phase 3 CARMEN-LCO3 trial, which led to the discontinuation of the study drug by the manufacturer.

Pharmacists and health care providers can counsel around vitamin D supplements and natural intake to increase a child’s vitamin D consumption.