Psoriasis
Latest News

Latest Videos
CME Content
More News
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.

This indication is for adult and pediatric patients aged 12 and older with plaque psoriasis of the scalp and body.
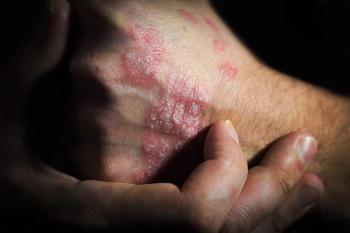
New trial results reveal icotrokinra's effectiveness in achieving significant skin clearance for challenging scalp and genital psoriasis.

The designation was supported based on data from the randomized, double-blind, active-controlled phase 3 interchangeability study.

The launch of ustekinumab-stba following its FDA approval expands patient access to this therapy with a variety of indications.

Icotrokinra, an oral pill that blocks the IL-23 receptor, had a favorable safety profile in adults and adolescents 12 years and older with moderate-to-severe plaque psoriasis.

Embedding a pharmacist in the dermatology clinic improved clinical and financial outcomes.
Through frequent interactions, pharmacists can discuss patients' treatment satisfaction, adherence issues, or potential adverse events.

Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.

If approved, the new indications include children 6 years and older with moderate to severe plaque psoriasis and children 5 years and older with active juvenile psoriatic arthritis.
Recently announced trial results demonstrate the efficacy of the biologic in multiple disease indications.

In April 2024, the FDA approved ustekinumab-aekn as a subcutaneous injection for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis.
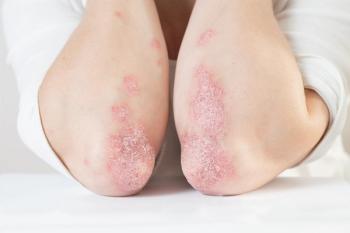
Additional education can address ambivalence for patients and providers, which can start at the pharmacist level, as they are essential sources of information for patients.
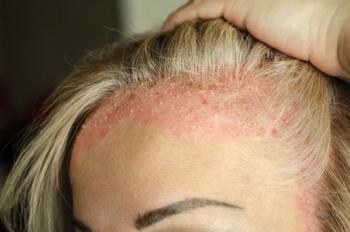
The FDA assigned a Prescription Drug User Fee Act action date of May 22, 2025.

The study authors recommend that health care professionals are aware of the connections, notably in adolescent patients and those with moderate to severe psoriasis.

Ustekinumab-ttwe (Pyzchiva; Sandoz) is approved for all of the same indications as Stelara.
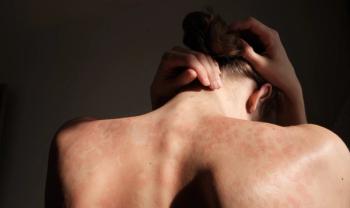
These options are typically more cost-effective, enhancing patient accessibility
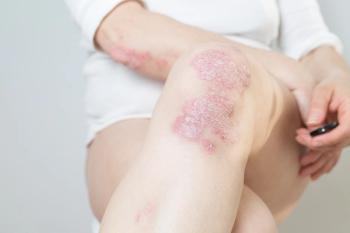
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.

To date, this is the only targeted therapy indicated for the treatment of generalized pustular psoriasis.

Compared to placebo, risankizumab significantly reduced symptom severity for patients with moderate to severe disease.

The tool consolidates and highlights key features that would be considered when choosing a biosimilar adalimumab.
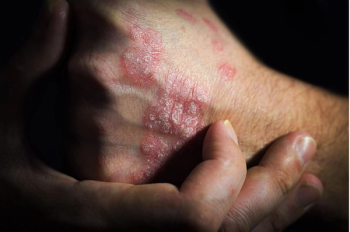
This treatment improved the severity of symptoms in patients with scalp and nail psoriasis.
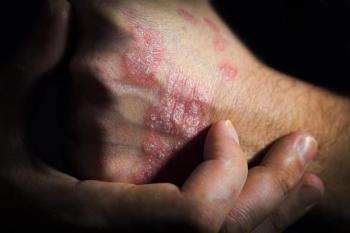
The biosimilarity was evident between SB17 (Samsung Bioepis), ustekinumab (Stelara; Janssen Immunology), and those switching from ustekinumab to SB17.

Adalimumab-ryvk is the first high-concentration, citrate-free biosimilar to Humira that has been granted interchangeability status for the 40 mg/0.4 ml injection.

Biologics for plaque psoriasis has been associated with an atopic dermatitis phenotype, paradoxical eczema, but the risk factors are not well known.