
The platform offers uninsured patients discounted drug prices, though it is important to note that the platform does not sell drugs, and only a small number are currently available.

The platform offers uninsured patients discounted drug prices, though it is important to note that the platform does not sell drugs, and only a small number are currently available.

In this episode, we break down the genetics, diagnosis, and clinical impact of HCM, explore how management strategies have evolved, and discuss the emerging role of cardiac myosin inhibitors in treating obstructive disease.


Morgan McSweeney, PhD, discusses why current US advisory structures are vulnerable to political influence, the vital role pharmacists play in sustaining vaccine confidence, and why universal hepatitis B vaccination remains essential in the US.

Morgan McSweeney, PhD, discusses the evidence behind the hepatitis B vaccine’s safety, how pharmacists can communicate with concerned parents, and the impact of shifting federal guidance on vaccine confidence.
The FDA approved mitapivat, the first oral therapy for thalassemia due to anemia, offering hope for patients with transfusion-dependent and non–transfusion-dependent forms.

The FDA approves Accrufer for children 10 and older, offering a well-tolerated solution for iron deficiency, enhancing pediatric health outcomes.


FDA approves Rybrevant Faspro, a groundbreaking subcutaneous therapy for EGFR-mutated lung cancer, enhancing patient comfort and survival rates.


Host Craig Beavers sits down with Marc Baines, cofounder and executive director of the HeartLife Foundation, to explore heart failure care through the lens of lived experience.

The FDA has approved a new combination therapy for BRCA2-mutated metastatic prostate cancer, showing significant efficacy in clinical trials.
As the new chair of the Advisory Committee on Immunization Practices (ACIP) signals interest in revisiting childhood and adolescent vaccine schedules, immunologist Morgan McSweeney, PhD, stresses that any changes must be anchored in decades of rigorous safety and efficacy data.

New research reveals that low cholesterol levels may indicate increased severity in Crohn disease, offering a potential new biomarker for monitoring inflammation.

New long-term data reveal avapritinib's impressive efficacy and safety in advanced systemic mastocytosis.

The Isa-VRd regimen shows consistent efficacy results across all age and frailty levels in patients with newly diagnosed multiple myeloma.
FDA approves Omisirge, the first cell therapy for severe aplastic anemia, offering hope for patients lacking donor matches and improving recovery rates.

Multidisciplinary approaches are crucial in treating complex cancer patients, focusing on tailored therapies and symptom management strategies.
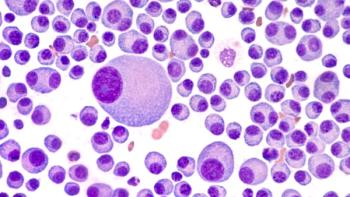
Pharmacists play a crucial role in optimizing CAR-T therapy outcomes by guiding bridging therapy decisions and enhancing patient safety through proactive collaboration.

New research reveals that effective bridging therapy before cilta-cel infusion significantly enhances safety and long-term outcomes in patients with multiple myeloma.


The American Society of Hematology (ASH) Annual Meeting and Exposition will feature the latest news, clinical trial updates, and key opinion leaders in the hematology field.

Although highly efficacious, patient selection and adverse event management are crucial.



Sam Klempner, MD, discusses early findings for the bispecific T-cell engager ASP2138, including monotherapy response, combination activity, and safety enhancements through subcutaneous dosing.
The combination has the potential to redefine the standard of care for a population that previously had limited therapeutic avenues.

Diana Isaacs, PharmD, discusses evolving perceptions of obesity, the expanding role of pharmacotherapy, and how pharmacists can drive access, counseling, and long-term metabolic health.

Published: July 14th 2023 | Updated:

Published: February 22nd 2023 | Updated:

Published: April 23rd 2025 | Updated:

Published: May 6th 2025 | Updated:
Published: December 24th 2025 | Updated: