
Osteoporosis
Latest News
Latest Videos
CME Content
More News

The approval of 2 denosumab biosimilars, Bildyos and Bilprevda, enhances access to affordable bone health treatments for patients.

The new approval expands the portfolio of denosumab biosimilars, increasing access and improving costs for patients.
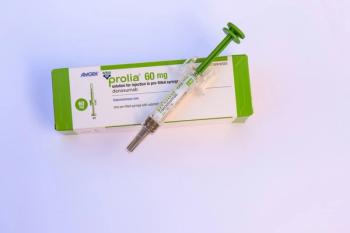
The approval in multiple indications is designed to improve access to effective, proven treatments for skeletal fractures, which can greatly reduce patient quality of life.
Through similar targeting of the RdRp enzyme, these treatments could offer groundbreaking new options for patients with COVID-19 if proven effective in real-world trials.

Investigators presented additional data from a clinical trial at the American College of Rheumatology Convergence, demonstrating similar safety for postmenopausal women with osteoporosis.

The non-opioid drug can induce healing of joint cartilage in patients with osteoarthritis.
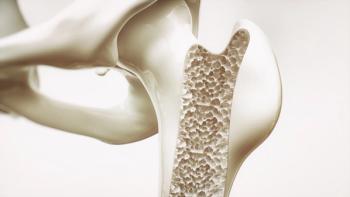
The use of intravenous immunoglobulin led to positive protective effects on osteoporosis.
The new findings were presented as an oral presentation at the 2024 European Calcified Tissue Society Congress, held from May 25, 2024, to May 28, 2024.

FKS518 (Fresenius Kabi) would be indicated for the treatment of osteoporosis in men and women, including glucocorticoid-induced osteoporosis and bone loss due to prostate or breast cancer.
Continued doses of denosumab reduced the incidence of diabetes by more than 1000 patients compared to an initial dose alone.
According to the FDA review, patients with CKD receiving denosumab for osteoporosis who are on dialysis or have CKD-MBD are at the highest risk of severe hypocalcemia.
Ten quiz questions to assess your knowledge on common osteoporosis treatments.

The lower a person’s T-score, the lower the bone density.

Patient education is a critical component of helping patients manage this common chronic condition.

The new drug application for PF708 was submitted under the 505(b)(2) regulatory pathway with teriparatide injection as the reference drug.
Amgen is indicated to treat osteoporosis in postmenopausal women at high risk for fracture.

Approved by the FDA on April 9, 2019, Amgen’s romosozumab-aqqg therapy (Evenity) is indicated for osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.

Top news of the day from across the health care landscape.

Romosozumab-aqqg (Evenity, Amgen) works to decrease the risk of fracture by increasing new bone formation in women with postmenopausal osteoporosis.

Romosozumab-aqqg (Evenity, Amgen) works to decrease the risk of fracture by increasing new bone formation in women with postmenopausal osteoporosis.

Top news of the day across the health care landscape.
Abaloparatide and teriparatide are the only anabolic agents approved by the FDA for women with osteoporosis at high-risk of fragility fractures.
Persistent use of osteoporosis medications was associated with reduced risk of fracture and significantly lower total healthcare costs, a recent analysis of Medicare claims data found.
The United States Preventive Services Task Force (USPSTF) updated its 2011 recommendation about osteoporosis screening.





























