Weight Management
Latest News

Latest Videos

Shorts
Podcasts
CME Content
More News

The FDA approved oral semaglutide to reduce the risk of major adverse cardiovascular events (MACE) in patients with type 2 diabetes (T2D).
The glucagon-like peptide-1 (GLP-1) receptor agonist medications evaluated included semaglutide, tirzepatide, and liraglutide.
A tour through GLP-1’s unexpected biology.
Explore the differences between oral and injectable semaglutide for weight management, focusing on adherence, adverse effects, and patient preferences.
The pivotal clinical trial supports the concomitant administration of tirzepatide and ixekizumab, which significantly improves outcomes for patients with psoriatic arthritis (PsA) and obesity.

Recent studies reveal that weight recovery in anorexia nervosa does not ensure muscle restoration, highlighting the need for comprehensive recovery strategies.

New oral GLP-1 agonist orforglipron cuts A1C and drives meaningful weight loss in trials, offering easier dosing than injections.

GoodRx enhances access to semaglutide with affordable cash pricing, revolutionizing obesity treatment and supporting patients in weight management.

Orforglipron shows promise in maintaining weight loss for individuals transitioning from injectable therapies, offering a convenient oral option for obesity management.

The FDA approved the first oral GLP-1 pill for weight management, offering a convenient alternative for obesity treatment and enhancing patient access.
Retatrutide shows promising results in reducing weight and knee pain for individuals with obesity and osteoarthritis, enhancing physical function significantly.

Pharmacists debate the effectiveness and ethics of GLP-1 agonists for pediatric obesity, highlighting the need for balanced treatment approaches.

GLP-1 receptor agonists expand treatment options for diabetes, weight loss, and more, showing promise in heart failure, kidney disease, and neuroprotection.
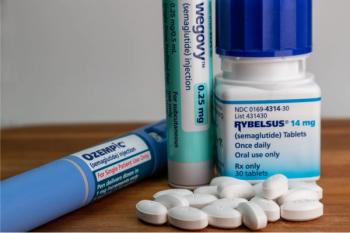
The 1-year extension period in trials will be discontinued based on the efficacy results observed in the overall study population, said Novo Nordisk.
Plozasiran gains FDA approval to significantly lower triglycerides in adults with familial chylomicronemia syndrome, enhancing heart health management.

Semaglutide shows significant weight loss and reduces obesity-related health risks, offering a promising solution for effective weight management.
The move continues the push towards most favored nation (MFN) status, lowering the prices of glucagon-like peptide-1 (GLP-1s) drugs for Medicare recipients.

Oral semaglutide shows promise in enhancing glucose control and reducing cardiovascular risks.

Berberine is increasingly used for weight management despite limited supporting evidence, emphasizing the need for pharmacist-led education, interaction monitoring, and adverse effect counseling.

Recent research reveals rising cancer rates among younger and older adults, linking obesity to increased incidence.
Lawmakers weigh cost concerns, clinical outcomes, and regulatory barriers as access to these medications evolves.
Pharmacists are uniquely positioned to help prevent nutritional deficiencies, manage adverse effects, and maintain long-term health through tailored guidance.

GLP-1 receptor agonists show potential as innovative treatments for alcohol and substance use disorders, addressing critical public health needs.