Overall, approximately 41% of those receiving Axi-cel and 16% of those receiving standard of care survived for 2 years without needing additional cancer treatment or experiencing cancer progression.

Overall, approximately 41% of those receiving Axi-cel and 16% of those receiving standard of care survived for 2 years without needing additional cancer treatment or experiencing cancer progression.
The standard-of-care for patients with LBCL consists of chemotherapy and additional high intensity chemotherapy followed by stem cell transplantation.

Closing out their discussion on marginal zone lymphoma, the panel considers which future treatment strategies are most promising.
According to the investigators, in vitro functional studies demonstrate potent antitumor activity of ATA2271 following repeat antigen stimulation.

Moving beyond BTK inhibition in marginal zone lymphoma, expert panelists review other therapies and drug classes with indications in this setting.
Following the success of ibrutinib, second-generation BTK inhibitors have shown even better outcomes with fewer toxicities.
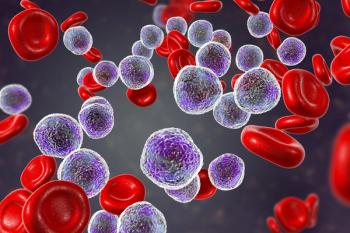
The body of research around acalabrutinib is becoming quite advanced, allowing clinicians to feel confident when treating patients.

New data are being presented at ASH 2021 regarding the durable efficacy of acalabrutinib in chronic lymphocytic leukemia.

The multicenter phase 3 study randomized patients with R/R CLL 1:1 to receive acalabrutinib 100 mg orally twice daily or investigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab until disease progression or unacceptable toxicity.
The combination therapy of ibrutinib and venetoclax was superior to chlorambucil and obinutuzumab in terms of undetectable minimal residual disease responses in elderly or unfit patients with previously untreated chronic lymphocytic leukemia.

Daratumumab continued to demonstrate deep and durable responses, including improvements to stringent complete response (sCR) rates and minimal residual disease (MRD)-negativity.
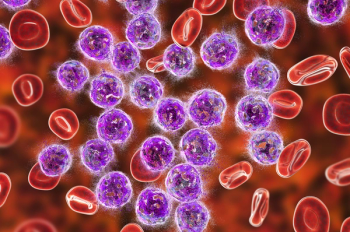
Axicabtagene ciloleucel (Yescarta) was the first CAR T-cell immunotherapy to be approved for relapsed or refractory large B-cell lymphoma.

Factor V Leiden can increase the chance of developing abnormal blood clots, most commonly in the legs or lungs.
Rylaze is a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphoma.

Panelists look at the class effects of BTK inhibitors and share optimal strategies to select therapy and manage toxicities.

Expert perspectives on the MAGNOLIA trial and novel BTK inhibitor zanubrutinib in the context of marginal zone lymphoma treatment.

FDA to evaluate luspatercept-aamt (Reblozyl) for the treatment of anemia in adults with non–transfusion dependent β-thalassemia.

FDA approves rituximab (Rituxan) plus chemotherapy for pediatric patients with previously untreated, advanced stage, CD20-positive diffuse large B-cell lymphoma, Burkitt lymphoma, Burkitt-like lymphoma, or mature B-cell acute leukemia.
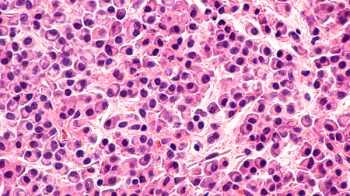
Ibrutinib in combination with venetoclax shows promise in patients with previously untreated chronic lymphocytic leukemia.

The combination of daratumumab with hyaluronidase (Darzalex Faspro), hyaluronidase-fihj, carfilzomib (Kyprolis), and dexamethasone is indicated for the treatment of adult patients with relapsed/refractory multiple myeloma who have received 1 to 3 prior lines of therapy.

Further, at daily doses of at least 100 mg, the rates of VTE with milvexian were significantly lower than with enoxaparin, according to the study authors.

A broad review of BTK inhibition as a novel therapeutic approach toward the treatment of marginal zone lymphoma.

Following discussion on the NCCN guidelines for second-line therapy in marginal zone lymphoma, experts highlight approved treatment options.

Besermi is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera.
Within 1 or 2 days of injury, therapeutic B cells release a complex mix of specific pro- and anti-inflammatory molecules, which can affect molecular processing and facilitate healing.