The panel also recommends considering risk factors for poor response, including uncontrolled disease, immunoparesis, the number of previous lines of therapy, and patient age.

The panel also recommends considering risk factors for poor response, including uncontrolled disease, immunoparesis, the number of previous lines of therapy, and patient age.
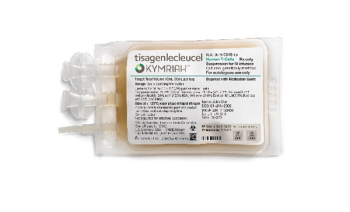
Kymriah is a CD19-directed genetically modified autologous T-cell immunotherapy.

No new safety concerns were encountered, which the investigators said support the frontline use of daratumumab plus lenalidomide and dexamethasone for patients with multiple myeloma who are ineligible for transplantation.

Jennifer Andrews, MD, pediatric hematologist-oncologist at Vanderbilt University Medical Center and medical director of Vanderbilt University Medical Center’s blood bank, discusses what health systems can do to address the national blood shortage and increase blood donations.

Ciltacabtagene autoleucel (cilta-cel) CAR T-cell therapy is currently being evaluated for the treatment of patients with relapsed and/or refractory multiple myeloma.
Deep vein thrombosis can occur with certain medical conditions that affect how the blood clots.
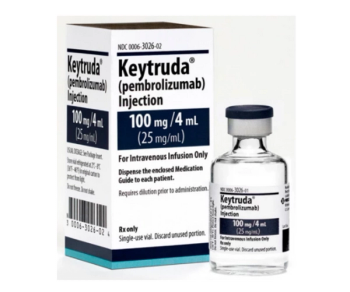
Pembrolizumab (Keytruda) can be used for multiple cancer types, including melanoma, non-small cell lung cancer, and head and neck squamous cell cancer.

Asciminib (Scemblix) approved for patients with Philadelphia chromosome–positive chronic myeloid leukemia (CML) in chronic phase who were previously administered 2 or more tyrosine kinase inhibitors.
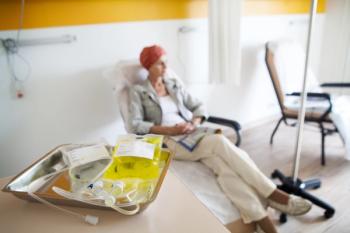
Patients undergoing chemotherapy had lower COVID-19 antibody levels than patients undergoing targeted therapy following vaccination.
Jennifer Andrews, MD, pediatric hematologist-oncologist at Vanderbilt University Medical Center and medical director of Vanderbilt University Medical Center’s blood bank, discusses how a national blood shortage could affect patient care and outcomes.

Jennifer Andrews, MD, pediatric hematologist-oncologist at Vanderbilt University Medical Center and medical director of Vanderbilt University Medical Center’s blood bank, discusses what the national blood shortage may mean for health systems.

Avacopan (Tavneos, ChemoCentryx) met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks during a phase 3 trial in patients with severe active anti-neutrophil cytoplasmic autoantibody-associated vasculitis.

Isatuximab-irfc (Sarclisa; sanofi-aventis) provides patients with another option for treatment and significantly reduces the risk of disease progression or death.

A single infusion of the CAR T-cell therapy Tecartus produced a high and durable response rate in heavily pretreated patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia.

Among 38 patients with B-cell derived malignancies who were seronegative after 2 COVID-19 vaccine doses, 55% had detectable antibodies after the third dose whereas 45% remained seronegative.
Patients with relapsed or refractory chronic lymphocytic leukemia are generally divided by those exposed to Bruton's tyrosine kinase inhibitors (BTKi), patients relapsing after venetoclax, and patients relapsing after BTKi and B-cell lymphoma-2 inhibitors.

Robert Z. Orlowski, MD, PhD, director of the Myeloma Section at the University of Texas MD Anderson Cancer Center, reviewed novel agents in the treatment of triple-class refractory myeloma during a session at the 2021 annual meeting of the Society of Hematologic Oncology (SOHO).

The recommendation for managing cytopenias among patients treated with tyrosine kinase inhibitors is to discontinue treatment at the onset of grade 3 or higher.

Risk stratification for survival should not dictate treatment, but stratification for thrombosis risk should play an important role in treatment decisions.
By deactivating a component of the MAPK signaling pathway, researchers were able to reduce the proportion of leukemia cells in blood and bone marrow.

Andrea Silber, MD, said the COVID-19 pandemic has exacerbated disparities that already existed within oncology care, and she said the effects of these disparities on patients are very real and direct.
Crizanlizumab is a humanized IgG2 kappa monoclonal antibody that binds to P-selectin and blocks interactions with its ligands.
A presentation at the Society of Hematologic Oncology 2021 Annual Meeting reviewed the treatment of amyloidosis in multiple myeloma.
Shereen Stutz and Mark Alwardt, of McKesson, said the rise in oral oncolytics during the COVID-19 pandemic allowed pharmacists to be more innovative and to become an even more integrated part of the oncology care team.

Patients now have multiple options, including continuous therapy with BTK inhibitors and fixed-duration therapy with venetoclax-based regimens.