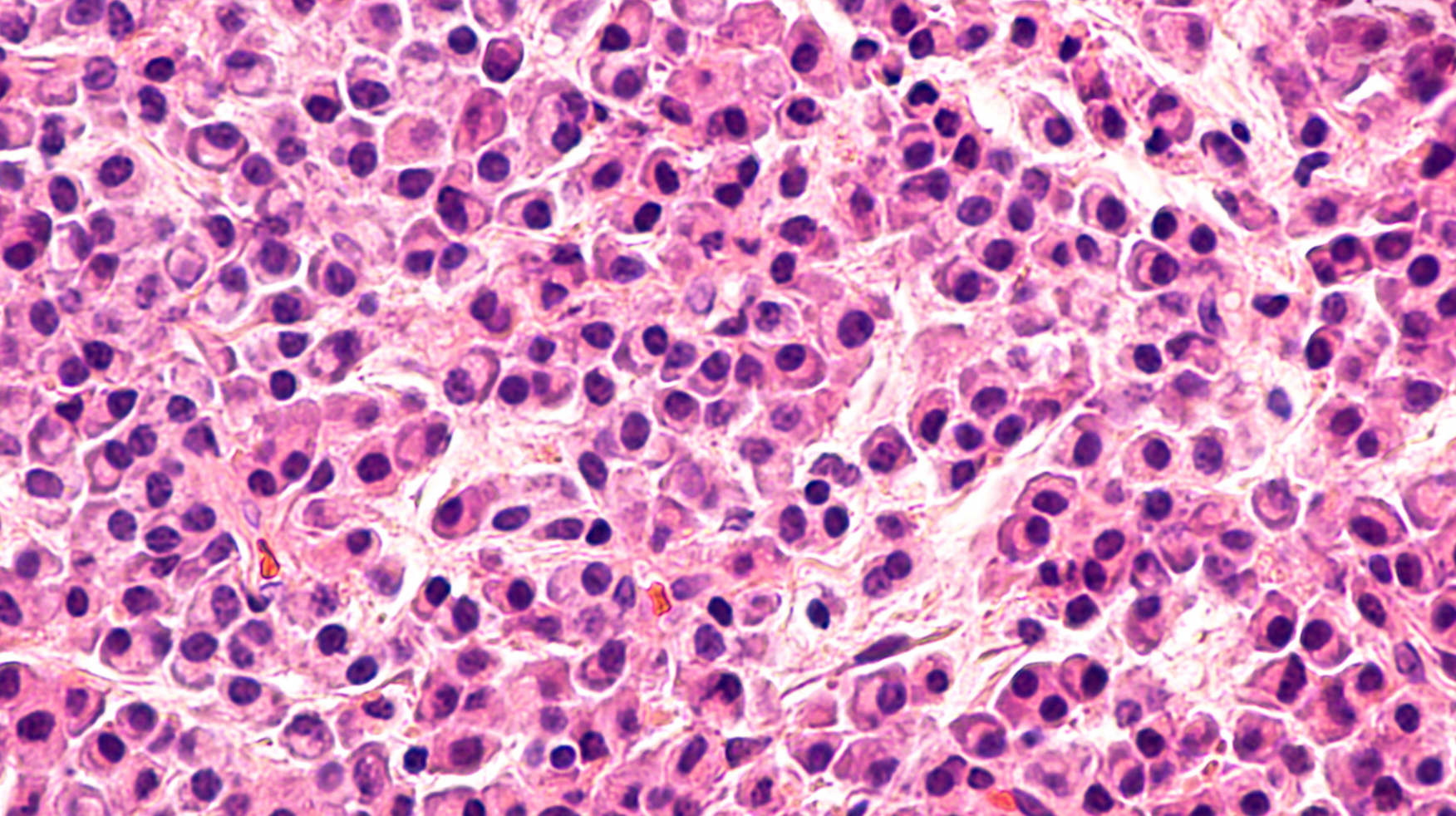
The addition of daratumumab to lenalidomide and dexamethasone resulted in a significant benefit to overall survival in patients with newly diagnosed multiple myeloma who were ineligible for autologous stem cell transplant and were treated to progression, according to the results of the phase 3 MAIA study.