
This is an update to the 2011 AAN guideline on the treatment of painful diabetic neuropathy, according to a press release.

This is an update to the 2011 AAN guideline on the treatment of painful diabetic neuropathy, according to a press release.

When using a therapy to clear the senescent cells, disease progression and cell death were stopped, according to the study.

Nurtec is the first oral calcitonin gene-related peptide antagonist approved for both acute and preventative treatment of migraine.
Inhibiting the activity of group 3 innate lymphoid cells could serve as a new therapeutic approach for neurologic conditions.

Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses the problems with approaching public health issues, such as mental health and substance use disorders, with an industrialized approach to problem solving.

Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses whether the adoption and acknowledgement of psychedelic medicine in health care systems looks likely for the future.

Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses which environments each psychedelic medicine is likely to be administered.
Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses which psychedelic drugs are currently under investigation for potential FDA approval.
David Olson, PhD, the chief innovation officer, head of scientific advisory board, and co-founder of delix therapeutics and an associate professor at University of California, Davis, discusses psychedelics and other psychoplastogens in the treatment of brain and mental health disorders.
A panel of experts discuss the future of psychedelic medicine and the role of the pharmacy in that future.
Gabapentin enacarbil treats moderate-to-severe primary restless leg syndrome and postherpetic neuralgia.
Saol Therapeutics also announces the acquisition of 4 plasma-derived hyperimmune products by Kamada, which will be invested to help launch Lyvispah and other pipeline developments.
About 74% of individuals who remained on cenobamate had early-onset responder rates and high rates of sustained seizure reduction for as long as 43 months, study results show.

Febrile seizures occur in young, healthy children with normal development.

Ten quiz questions to assess your knowledge on common symptoms and treatments for Alzheimer disease.
Investigators have developed an antibody-based treatment for Alzheimer disease, as well as a protein-based vaccine.
Ron Aung-Din, MD, the clinical medical advisor of Pyscheceutical, and Chad Harman, the CEO of Pyscheceutical, discuss a novel approach for administering psychedelics for the treatment of brain disorders that does not induce hallucinogenic effects.

Diagnosis, treatment options, and the role of the pharmacist in patient care are all important factors when looking at multiple sclerosis as a whole.
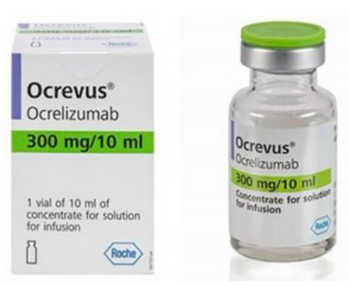
Ocrelizumab is indicated for the treatment of relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive disease, in adults.
Investigators from Michigan Medicine analyzed data from 16,700 individuals in a new study.
Tosymra is indicated for the acute treatment of migraine with or without aura in adults.

The guidance updates the recommendations on dopaminergic medications that were published in 2002 on the initiation treatment for Parkinson disease.
Approximately 40% of patients with stage IV melanoma have brain metastases at diagnosis.

Aducanumab-avwa is a human, immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble and insoluble forms of amyloid beta.