
Agency gives green light to supplemental new drug application for repository corticotropin injection.

Agency gives green light to supplemental new drug application for repository corticotropin injection.

Atogepant is indicated for the preventive treatment of episodic migraine in adults.
According to the investigators, these new data support ofatumumab as a first-choice treatment for adult patients with RMS, including those who are newly diagnosed.
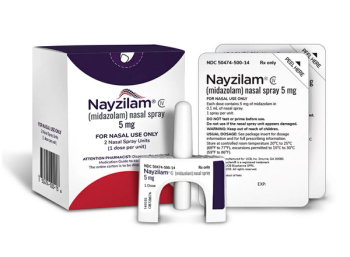
Midazolam is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern in patients with epilepsy.

Gyang and Hansen discussed any new or existing treatment options for patients, the role of the pharmacist changing, and what they wish others knew about MS.
An effective therapeutic treatment for acute or chronic pain needs to be convenient, easy to self-administer, and have rapid onset to provide fast relief.

Pharmacy Times spoke with neurologist Dr. Tirisham Gyang and pharmacist Dr. Margaret Hansen about multiple sclerosis, including diagnosis, treatment options, and the role of the pharmacist in patient care.

Treatment options for autism spectrum disorder may include selective serotonin reuptake inhibitors, tricyclics, antipsychotics, stimulants, anti-anxiety medications, and anticonvulsants.
Early intervention with ocrelizumab resulted in a 35% reduction of risk in individuals with RMS who needed a walking aid over 7 and a half years.
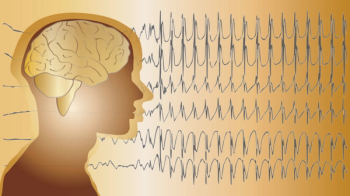
Types of seizure include focal seizures with impaired awareness, generalized seizures without loss of consciousness, and generalized seizures, such as absence seizures.
Viruses that have been linked to Bell's palsy include those that cause cold sores and genital herpes (herpes simplex); Chickenpox and shingles (herpes zoster); and infectious mononucleosis.
Those who are younger than aged 40 years tend to regain these senses at higher rates than those who are older.
Nusinersen (Spinraza) is indicated for the treatment of spinal muscular atrophy (SMA) in pediatric and adult patients.

Glatiramer acetate modifies immune processes that are believed to be responsible for the pathogenesis of multiple sclerosis.

Significant reductions in mean monthly migraine days compared with placebo were seen as early as weeks 1 through 4 in the clinical trial program for atogepant.
Approximately 5.8 million people in the United States 65 years of age and older live with Alzheimer disease.
Although frontotemporal dementia is the leading cause of the disease for those under 65 years of age, far fewer people are familiar with it than with Alzheimer disease.

ALS results in muscles that are reduced in size (atrophic), weak, and soft, or muscles that are stiff, tight, and spastic.
Migraine headaches can be caused by triggers such as hormonal changes, drinks, stress, sensory stimuli, sleep changes, physical factors, weather changes, medications, foods, and food additives.
In MS, the immune system attacks the protective sheath (myelin) that covers nerve fibers, causing deterioration of the nerves.

Research results show a possible link between herpesviruses and neurodegenerative diseases, such as Alzheimer disease, ALS, and glaucoma.
The nasal spray bypasses the gut and potential absorption issues associated with it, and offers consistent relief even when administered hours after the onset of a migraine attack.

A greater proportion of atogepant-treated participants achieved at least a 50% reduction in mean monthly migraine days.

The study authors note that they cannot conclude that there is a causal relationship between the COVID-19 vaccines and any of the individual incidents of Bell palsy reviewed.

The FDA's green light for the Alzheimer medication is worrisome, as clinical trials proved inconclusive.