
With the help of pharmacists and a team of health care providers, a better quality of life is possible for patients with Parkinson disease.

With the help of pharmacists and a team of health care providers, a better quality of life is possible for patients with Parkinson disease.

Researchers find that the risk of developing dementia increased by 35% in patients with atrial cardiopathy.

Seizalam is a benzodiazepine indicated for the treatment of status epilepticus in adults.
As local drug delivery technology evolves, the field is set for monumental change.

Tardive dyskinesia is often caused by the use of dopamine receptor blocking agents, most commonly antipsychotics, for at least a few months.

A recent study revealed that social activities like gardening or getting a college degree could improve cognition in older age, which could possibly prevent Alzheimer disease.
Currently, there is no treatment targeted for superoxide dismutase 1 amyotrophic lateral sclerosis, which has a life expectancy of 3 to 5 years from time of symptom onset.
They can assess for adherence, identify drug-related problems, monitor pharmacotherapy, and provide education.

Researchers highlight how a lack of access to medications used to treat multiple sclerosis, migraines, and epilepsy, but not proven safe for pregnancy, threatens child-bearing aged women who live with these conditions.

Zonisade is the first and only FDA-approved oral liquid formulation of zonisamide, which helps patients with epilepsy who have difficulty swallowing tablets.

Apomorphine (Kynmobi) is indicated for the acute, intermittent treatment of off episodes in patients with Parkinson disease.
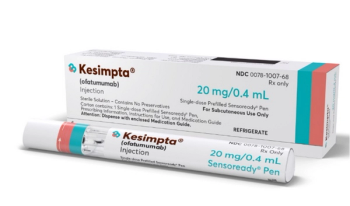
Ofatumumab (Kesimpta) is a CD20-directed cytolytic antibody indicated for the treatment of relapsing forms of multiple sclerosis.

Brexpiprazole is indicated as an adjunctive therapy to antidepressants in adults with major depressive disorder and as a treatment in adults with schizophrenia.
After 4 years of treatment with ofatumumab, 78.8% of patients with relapsing forms of multiple sclerosis who continuously received ofatumumab achieved NEDA-3 compared to 51.8% of those who switched from teriflunomide to ofatumumab.
Jazz Pharmaceuticals announced top-line results from the phase 3 RELEASE MSS1 trial, evaluating nabiximols oromucosal spray in individuals with multiple sclerosis.

Surprising turn of events is a strong message to the pharmaceutical industry about the acceptable developmental pathway for this drug class.
New data show that after 4 years of treatment, 78.8% of patients who continuously received ofatumumab achieved no evidence of disease activity-3 (NEDA-3) compared to 51.8% of those who switched from teriflunomide to ofatumumab.

Cenobamate is a once daily anti-epileptic medication with a recommended maintenance dose of 200 mg once daily that can be prescribed as monotherapy or adjunctive therapy.

Patients recorded their symptoms and use of acute medications in a headache diary.
Levetiracetam is an anticonvulsant used to treat epileptic seizures.
Natalizumab (Tysarbi) is an integrin receptor antagonist indicated for treatment of: multiple sclerosis and Crohn disease.

The symptoms of serotonin syndrome often include autonomic dysfunction, neuromuscular excitation, and altered mental status.

Specialty pharmacy is deeply engaged in providing treatment for conditions that are at high risk for mental health challenges.
Naxitamab-gqgk (Danyelza) is for the treatment of pediatric and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable disease to prior therapy.

Studies have estimated that 16% of people with dementia experience depression, but this may be as high as 40%, demonstrating a great need for effective treatments.