Immuno-oncology
Latest News
Latest Videos

CME Content
More News
Overall survival results from the phase 3 KEYNOTE-522 study provide evidence of improved long-term outcomes from this pembrolizumab-based regimen.
Clinical research presented at ESMO 2024 highlights the significant survival benefits of perioperative and neoadjuvant immunotherapy with nivolumab and nivolumab plus ipilimumab, while emphasizing the role of biomarkers such as ctDNA and KRAS mutations in guiding treatment decisions.
An ESMO 2024 presentation explores the growing role of the gut microbiome in immunotherapy response, emerging diagnostic tools such as PCR chips, and innovative therapies such as fecal microbiota transplantation and microbiome-preserving strategies to improve patient outcomes.
Christian Rolfo discusses 6 different treatment options that are being developed across immunotherapies and antibody drug conjugates for non–small cell lung cancer and small cell lung cancer.
Subcutaneous administration of atezolizumab and hyaluronidase-tqjs had similar efficacy to intravenous administration.
Highlights from multiple trials investigating novel treatments for mNSCLC were presented at 2024 World Conference on Lung Cancer.

Breanne Peyton-Thomas, PharmD, BCOP, discusses her work as a pharmacist at Ochsner Health on the CAR T-cell therapy team.
Stefan Barta, MD, MS, MRCPCUK, discusses the potential role of biomarkers in predicting treatment outcomes, the emerging use of CAR T-cell therapy, and the benefits and challenges of combination therapies in managing T-cell lymphoma.
Sonali Smith, MD, discusses T-cell–directed therapies for indolent B-cell lymphoma, focusing on their efficacy, FDA-approved treatments, and treatment challenges, such as managing cytokine release syndrome.

Mary McGann, PharmD, BCOP, offers insights into CAR T-cell therapy and the crucial role of pharmacists in mitigating resistance.
The data builds off prior studies supporting bendamustine-rituximab as a first-line treatment for patients with non-Hodgkin lymphoma.
The trial is evaluating the success of BNT111 and cemiplimab in treating unresectable stage III or IV melanoma whose disease had progressed following anti-PD-(L)1-containing treatment.
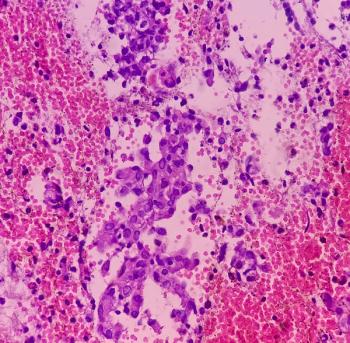
The RUBY trial will continue and analyze the overall population survival after treatment with the drug combination.
Treatment of advanced/metastatic urothelial cancer has changed dramatically in recent years. This review discusses the use, risks, and benefits of several novel therapies for this indication.

Collaboration between community centers and academic medical centers can improve patient access to these complex therapies.

In Iraq, asymmetrical warfare tactics relied heavily on unconventional weaponry, requiring military physicians to improvise new medical techniques in order to increase rates of survival.

Kirollos Hanna, PharmD, BCPS, BCOP, FACCC, discusses the role of the pharmacist in relation to bispecific care team readiness and patient readiness.
The results displayed a dramatic reduction in the individuals’ tumors within days after single treatment.

Results from the phase 3 RATIONALE 302 trial showed tislelizumab-jsgr prolonged survival compared to chemotherapy in patients who received prior systemic treatment.

Less manufacturing time could be the difference between a patient receiving this life-saving therapy vs not.
The immune checkpoint inhibitor increased inflammation within the tumor, which may be linked to better response to nivolumab treatment.
The candidate has already demonstrated anti-tumor activity in chemotherapy-naïve patients.

The CAR T-cell therapy improved relative overall survival by more than a third compared to standard of care.
As the use of sequencing, proteomics, and immunopeptidomics increases, more targets will also be discovered, as well as more biomarkers that predict responses to treatments.

Pembrolizumab (Keytruda) gains its sixth approval in non–small cell lung cancer (NSCLC), with the latest indication in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a monotherapy for the post-surgical adjuvant treatment of patients with resectable NSCLC.