Biosimilars
Latest News
Latest Videos

CME Content
More News
Pharmacogenomics is gaining support in mental health and polypharmacy, where the data make a strong case for improvements in clinical and economic outcomes.

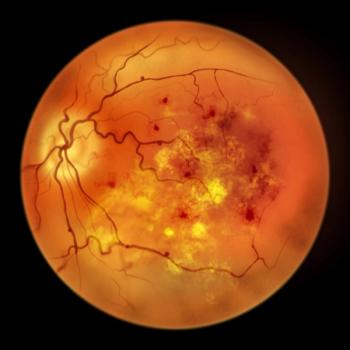
SB15 had a comparable safety, pharmacokinetics, and immunogenicity treatment profile to AFL for age-related macular degeneration.

Upadacitinib 15 mg once daily had a better clinical response compared to adalimumab 40 mg every other week at 12 weeks in 3-year follow-up data among patients with rheumatoid arthritis.

As more becomes known about Crohn disease, treatment modalities have evolved with biologic monoclonal antibodies becoming a key part of guideline- and evidence-based medicine.

With so many radical developments in medicine and technology unfolding at once, the role and potential of the pharmacy as we know it is evolving at breakneck speed.

It is the second anti-TNFα biosimilar approved for use in the US.

In a single-center study, investigators reviewed charts of 25 children with inflammatory bowel disease transitioning from infliximab to the biosimilar infliximab-dyyb for maintenance.

Adalimumab-adbm (Cyltezo Pen; Boehringer Ingelheim) is approved by the FDA as an interchangeable biosimilar to Humira.

Pharmacists should continue to monitor payer policy changes and reimbursement rates and improve understanding of electronic order entry.
Pegfilgrastim-pbbk is a leukocyte growth factor developed to reduce the onset of infection in patients with non-myeloid malignancies administered myelosuppressive anticancer medications associated with a clinically significant rate of febrile neutropenia.

Expert: Though Patients Don’t Always See Savings With Biosimilars, New Policies Could Improve Access
Fran Gregory, PharmD, MBA, vice president of Emerging Therapies at Cardinal Health, discussed the role biosimilars are playing in the pharmaceutical market as well as in patient access.

As patents expire, the development of biosimilars may increase, opening up new opportunities to compare efficacies of these drugs.

Gregory also discussed the differences between specialty pharmacy distribution and physician’s office distribution, and the benefits of each for patients.
Biosimilars are also expanding rapidly, offering new opportunities for payers, patients, and providers.
Pharmacists will play a growing role in the use of biosimilars and guiding patients through the switching process.

Fran Gregory, PharmD, MBA, vice president of Emerging Therapies at Cardinal Health, discussed the biosimilars landscape and where biosimilars fall in the process of pharmacy and medical benefit adoption.

Balancing clinicians’ priorities with the needs of patients and their families is crucial to ensure access and adherence to medications.

The willingness of biology labs to evolve has been essential to the incredible rise of biologic drugs.

As Biosimilars Change the Payer Landscape, Pharmacists Can Help Patients Access, Manage Complexities
Cate Lockhart, PharmD, PhD, executive director of the Biologics and Biosimilars Collective Intelligence Consortium, discussed the growing biosimilars landscape .

Choosing the best biosimilar based on reimbursement can be challenging.

Infusion reactions in the switching group did not occur with switching, but rather only with reference trastuzumab administration.

Annual Asembia summit features insights from key opinion leaders, networking events, and continuing education sessions for pharmacists, pharmacy staff and stakeholders.

The FDA’s temporary Guidance for 503A compounding of shortage drugs was meant to be temporary and withdrawn once COVID-19 ended.

Individuals who are rituximab-naïve remained stable after 2 years of follow-up, the analysis shows.

Part 1 of this 2-part series examines USP <797> and <795> revisions for sterile and nonsterile compounding, veterinary compounding, FDA inspections of 503A pharmacies, and the interstate distribution of compounded prescriptions post Wellness Pharmacy v. FDA.