Biosimilars
Latest News
FDA Approves Nufymco, Interchangeable Ranibizumab Biosimilar, for Retinal Diseases
Latest Videos

Podcasts
CME Content
More News

The FDA approves a new dose of Omlyclo, the first interchangeable biosimilar to Xolair, enhancing treatment options for allergic and inflammatory conditions.

The biosimilars can be used to treat osteoporosis and cancer-related bone loss in certain populations.

The FDA approves Poherdy, a new biosimilar to Perjeta, enhancing treatment options for HER2+ breast cancer patients with affordability and efficacy.

Razumab, a ranibizumab biosimilar, shows comparable efficacy to its innovator counterpart in treating myopic choroidal neovascular membrane (mCNVM), offering a cost-effective solution.

The FDA designates Stoboclo and Osenvelt as interchangeable biosimilars for denosumab, enhancing treatment options for osteoporosis and cancer-related conditions.

FDA designates Conexxence and Bomyntra as interchangeable denosumab biosimilars, enhancing access and affordability for patients needing critical therapies.
The agency announced that human clinical trials will no longer be required in lieu of a comparative analytical assessment to determine biosimilar equivalency with its reference product.

The mean time intervals between injection and complete retinal vascularization were similar between the biosimilar and its reference product.
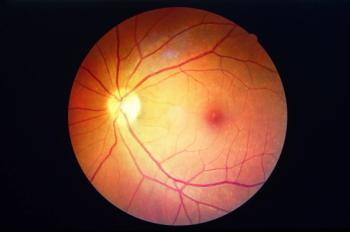
The FDA approves Eydenzelt, a biosimilar for retinal diseases, enhancing treatment options for diabetic macular edema and age-related macular degeneration.

Recent research confirms that rheumatoid arthritis patients in remission can safely switch to the biosimilar infliximab-dyyb, offering a cost-effective treatment option.
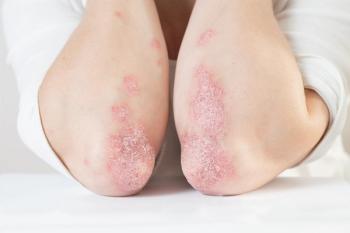
A phase 3 study shows SYSA1902, a biosimilar to Stelara, is clinically equivalent for treating moderate to severe plaque psoriasis.

Hercessi shows promising efficacy and safety in treating HER2+ metastatic breast cancer (MBC), with no significant risks from switching from Herceptin.

Additionally, the FDA granted a provisional interchangeability designation to both biosimilars.

Patients with inflammatory bowel disease (IBD) who switched to the biosimilar reported adverse events, but these are believed to stem from health anxiety.
Pavblu, a biosimilar to Eylea, also showed no clinically meaningful differences in efficacy, safety, and immunogenicity in patients with neovascular age-related macular degeneration (nAMD).

This decision is believed to redefine how biological drugs will be developed, approved, and made affordable for patients.

The approval of 2 denosumab biosimilars, Bildyos and Bilprevda, enhances access to affordable bone health treatments for patients.

Biosimilars can be a more affordable and accessible treatment option for patients with rheumatoid arthritis (RA) and psoriatic arthritis (PsA).
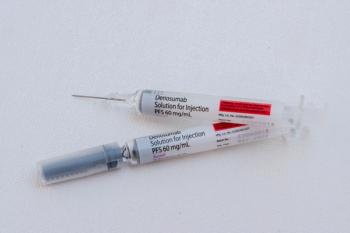
When treating patients with bone metastases, the biosimilar demonstrated equivalent safety and efficacy to its reference product.

A new biosimilar, LY05008, shows comparable efficacy and safety to dulaglutide in managing type 2 diabetes mellitus (T2DM) in Chinese adults.
New research confirms that switching from tocilizumab to its biosimilar, Avtozma, maintains efficacy and safety in patients with rheumatoid arthritis (RA).
Pharmacists optimize biosimilar and 505(b)(2) use through collaboration, education, and integration.
The FDA expands tocilizumab-anoh's approval for treating cytokine release syndrome (CRS), enhancing treatment options for patients aged 2 and older.

Herzuma shows promising efficacy and safety as a cost-effective biosimilar for treating HER2+ advanced gastric cancer, offering new hope for patients.
A recent study reveals Mvasi's comparable safety profile to Avastin for retinal diseases, highlighting the need for on-label biosimilars in ophthalmology.