Mononucleosis isn't as contagious as some infections, such as the common cold.

Investigators in a clinical study evaluating the safety, tolerability, and immunogenicity of the Pfizer-BioNTech COVID-19 vaccine in children aged 6 months to under 5 years noted that their research will continue with new changes.
Laura Lee Hall, PhD, president of the Center for Sustainable Health Care Quality and Equity, addresses disparities occurring in diabetes care among racial and ethnic populations.
A panel of experts discuss the future of psychedelic medicine and the role of the pharmacy in that future.

Study may help guide COVID-19 vaccination strategies for patients with blood disorders.

Researchers to explore possible approaches for designing small molecule therapeutics to combat depression.
Fetal alcohol syndrome causes brain damage and growth problems.

Study shows moderately reduced efficacy of the Moderna COVID-19 vaccine over time against Delta infection, which supports current booster dose recommendations.

Major metabolites for cannabinoids may interfere with two families of enzymes that help metabolize a wide range of drugs prescribed for a variety of conditions.

Health service planning involves a myriad of stakeholders to ensure their sustainability and their integration into other aspects of community health.

A single booster dose of the GlaxoSmithKline and Sanofi recombinant adjuvanted COVID-19 vaccine candidate delivered consistent and strong immune responses.

Neosporin prevents/treats only bacterial skin infections.
Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors.

After the initial infection, herpes virus lies dormant in the body and can reactivate several times a year.

The unique challenges that pharmacy learners encountered during the COVID-19 pandemic reinforce their importance in the professional pharmacy setting.

Pharmacists can help to reduce the prevalence of food insecurity and its negative health care outcomes by tailoring clinical care to each individual patient’s needs.

At week 16, 56.4% of patients receiving tofacitinib achieved an Assessment in SpondyloArthritis International Society 20 response.
Zofran aids in the prevention of nausea and vomiting.

Some minor skin wounds may heal faster when an antibiotic is applied to the affected area.
Gastrointestinal bleeding can range from mild to severe and can be life-threatening.

In specialty pharmacy, many patients take steps to manage their prescription benefits before the new year.
NRx Pharmaceuticals announces that no new concerns were identified by an independent data safety monitoring board, which recommends continued enrollment.
The findings are consistent with an interim analysis showing that the drug significantly reduced the risk of hospitalization or death for any cause by 89% compared with the placebo.

Technology, COVID-19, and scope expansion—here’s how pharmacists can capitalize on opportunities coming in the new year.

Offering individual advice and counseling to patients can help combat the risks of driving under the influence of prescription drugs.
The study examined safety, immune response (immunogenicity), and adverse effects of 7 vaccines when used as a third booster jab.
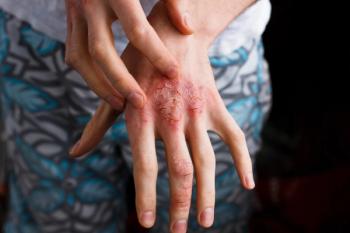
The data presented shows that at 16 weeks, individuals who added the atopic dermatitis drug to low potency standard-of-care topical corticosteroids experienced 28% achieved clear or almost-clear skin.

Dermoplast offers short-term relief of pain and itching from certain skin conditions, such as sunburn, scrapes, and insect bites.
GERD is mild acid reflux that occurs at least twice a week or moderate to severe acid reflux that occurs at least once a week.

The OTC Guide survey is open for pharmacists to participate through February 28, 2022, at 11:59 pm ET.