Tisotumab vedotin, is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.

Tisotumab vedotin, is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.
Ten quiz questions to assess your knowledge on common symptoms and treatments for prostate cancer.

Isatuximab-irfc (Sarclisa; sanofi-aventis) provides patients with another option for treatment and significantly reduces the risk of disease progression or death.
Ruxolitinib is indicated for intermediate or high-risk myelofibrosis, polycythemia vera, steroid-refractory acute graft-versus-host disease, and chronic graft-versus-host disease.

Oncology pharmacists can affect not only the efficacy of advancements in our health care system but also the scope of health care progress in our country.

The designation was based on data from the DESTINY-Breast03 phase 3 trial, and this is now the second BTD for trastuzumab deruxtecan in breast cancer, bringing its total number of BTDs to 4, according to an AstraZeneca press release.

A single infusion of the CAR T-cell therapy Tecartus produced a high and durable response rate in heavily pretreated patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia.
With a surge in development of oral oncolytic agents for a variety of therapy targets, the support pharmacists can provide patients in areas such as oral oncolytic medication adherence has come to the fore.

Prescribers, pharmacists, nurses, lab technicians, and other health care professionals must work as a team to create awareness and understanding of pharmacogenomics integration.
In an interview with Pharmacy Times®, Maria Whitman, Global Head of the Pharmaceutical and Biotech Practice at ZS, discusses the FDA’s Project Optimus, the FDA’s new guidance addressing dose optimization issues in oncology clinical trials, and how it has impacted drug manufacturers.

The investigators said these results suggest patients with low sTK1 activity levels have slow-growing disease, which can be initially controlled through single-drug endocrine therapy.
Margetuximab (Margenza) is indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer.

Among 38 patients with B-cell derived malignancies who were seronegative after 2 COVID-19 vaccine doses, 55% had detectable antibodies after the third dose whereas 45% remained seronegative.
Patients with relapsed or refractory chronic lymphocytic leukemia are generally divided by those exposed to Bruton's tyrosine kinase inhibitors (BTKi), patients relapsing after venetoclax, and patients relapsing after BTKi and B-cell lymphoma-2 inhibitors.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what the role of the pharmacist is during the treatment of patients with advanced cutaneous T cell lymphoma.

Robert Z. Orlowski, MD, PhD, director of the Myeloma Section at the University of Texas MD Anderson Cancer Center, reviewed novel agents in the treatment of triple-class refractory myeloma during a session at the 2021 annual meeting of the Society of Hematologic Oncology (SOHO).
The recommendation for managing cytopenias among patients treated with tyrosine kinase inhibitors is to discontinue treatment at the onset of grade 3 or higher.

The combination of cetuximab and encorafenib resulted in a median overall survival of 8.4 months, compared to 5.4 months in the control arm among patients with colorectal cancer.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses the areas that may need further investigation to progress advanced cutaneous T cell lymphoma treatments in the future.
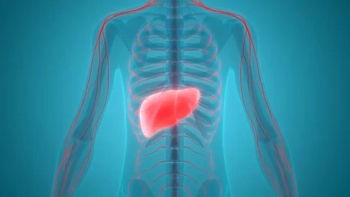
The medication has met the criteria of overall survival for individuals with this type of liver cancer who were previously treated with sorafenib, Merck says.

Risk stratification for survival should not dictate treatment, but stratification for thrombosis risk should play an important role in treatment decisions.

Cemiplimab-rwlc is being developed by Regeneron and Sanofi under a global collaboration agreement.

Durvalumab in combination with oleclumab reduced the risk of non-small cell lung cancer disease progression or death by 56%, whereas patients receiving durvalumab and monalizumab had a 35% reduction.
The combination maintained superior OS and response benefits versus sunitinib in both patients with intermediate- and poor-risk prognostic factors, the primary endpoint population, and across all randomized patients.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what research is underway in the advanced cutaneous T cell lymphoma field that she is currently keeping an eye on.
By deactivating a component of the MAPK signaling pathway, researchers were able to reduce the proportion of leukemia cells in blood and bone marrow.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, describes some of the challenges providers face during the treatment of patients with advanced cutaneous T cell lymphoma and how to address them.

Treatment with neoadjuvant cisplatin and pemetrexed plus atezolizumab for pleural mesothelioma found to be safe in trial.
Enfortumab vedotin-ejfv (Seagen; Padcev) is a first-in-class antibody-drug conjugate approved for patients with previously treated locally advanced or metastatic urothelial cancer.
The prostate-specific membrane antigen-targeted positron emission tomography imaging technique will be part of the National Comprehensive Cancer Network guidelines beginning in 2022.