Acalabrutinib is a Bruton tyrosine kinase inhibitor (BTKi) intended for the treatment of adult patients with mantle cell lymphoma who have received at least 1 prior therapy.

Acalabrutinib is a Bruton tyrosine kinase inhibitor (BTKi) intended for the treatment of adult patients with mantle cell lymphoma who have received at least 1 prior therapy.

Timely access to cancer treatment improves patient outcomes.
The investigators said that there were no new safety signals identified, and no reported instances of tumor lysis syndrome.

As some of the nation’s leading pharmaceutical and biotech giants ramp up product development and commercialization, what was once a futuristic concept has become a reality.
Ten quiz questions to assess your knowledge on common symptoms and treatments for endometrial cancer.
Tepotinib treats adult patients with metastatic non-small cell lung cancer harboring a mesenchymal epithelial transition exon 14 skipping alteration.
Genomic analyses show promise for patients with metastatic breast cancer, reinforcing genomics as a part of the pathway of care.

Tamoxifen is given to many patients—specifically premenopausal patients—with breast cancers that express the estrogen receptor, which drives breast tumor growth.

Pyrotinib combined with capecitabine found to improve overall survival in patients with previously treated HER2-positive metastatic breast cancer.
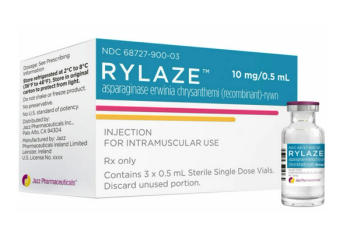
Rylaze is a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphoma.
In the case reviewed, the pharmacy department created an interdisciplinary Biosimilar Task Force responsible for making decisions relating to the adoption of biosimilar therapies.

FDA to evaluate luspatercept-aamt (Reblozyl) for the treatment of anemia in adults with non–transfusion dependent β-thalassemia.

FDA approves rituximab (Rituxan) plus chemotherapy for pediatric patients with previously untreated, advanced stage, CD20-positive diffuse large B-cell lymphoma, Burkitt lymphoma, Burkitt-like lymphoma, or mature B-cell acute leukemia.
Ibrutinib in combination with venetoclax shows promise in patients with previously untreated chronic lymphocytic leukemia.
Further, among premenopausal women, those who received chemotherapy had a 40% relative improvement in IDFS compared to those administered endocrine therapy alone.

Oncology pharmacists play a significant role in clinical pathways.

The combination of daratumumab with hyaluronidase (Darzalex Faspro), hyaluronidase-fihj, carfilzomib (Kyprolis), and dexamethasone is indicated for the treatment of adult patients with relapsed/refractory multiple myeloma who have received 1 to 3 prior lines of therapy.
The diagnostic tool widens the age group of individuals who can get tested and can be used in conjunction with a mammogram.

Underserved populations with limited access to medical care continue to be disproportionately impacted by certain cancers.
Olaparib was the first PARP inhibitor to be developed and has been the most studied.

The team found that out of more than 18,751 live births, 1008 cancer diagnoses were made in offspring 0 to 58 years of age.
Although specialty therapies have the potential to help people live healthier lives, their cost and complexity can create distinct challenges.

Ray Tancredi, RPh, MBA, divisional VP of specialty pharmacy development and brand Rx/vaccine purchasing at Walgreens, provides an analysis and overview of key specialty pharmaceuticals in development.

Survey results may imply that individuals who stop could have more health problems or die from a subsequent heart issue.

According to the researchers, almost half of the caregivers surveyed responded that the people they are caring for rely on them more than ever before, with 94% of respondents indicating that they put the needs of the person they care for above their own during the COVID-19 pandemic.

Besermi is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera.

Mobocertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR) that irreversibly binds to and inhibits EGFR exon 20 insertion mutations at lower concentrations than wild type (WT) EGFR.
The new sFIS assay may be capable of identifying immune biomarkers indicative of survival chances and treatment effectiveness for patients with ovarian cancer.
Patients with advanced renal cell carcinoma who were given the combination of nivolumab and ipilimumab demonstrated a significantly longer overall survival than those who received sunitinib.
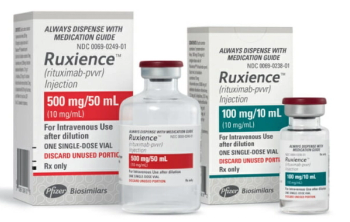
Rituximab-pvvr is indicated for the treatment of adult patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, and granulomatosis.