
Investigators have developed an immunotherapy that is a potential option for children with hard-to-treat brain cancer.

Investigators have developed an immunotherapy that is a potential option for children with hard-to-treat brain cancer.

The study’s identification of a network of possible candidate genes to carefully control for mutations could alter the future clinical implementation of CRISPR.
A survey of caregivers and parents of children with cancer shows that nearly one-third expressed hesitancy to vaccinate their youngsters against COVID-19.
Breast cancer found to be the most expensive type of the disease, costing approximately $3.4 billion in the United States.

Social determinants of health can play a significant role in the treatment trajectory and outcomes for patients with cancer because oncology care requires close communication between the patient and clinicians.

Study finds COVID-19 booster shot was extremely beneficial for all patients with cancer, especially those with a blood cancer.
Approximately 40% of patients with stage IV melanoma have brain metastases at diagnosis.
The results of a study showed that non-Hispanic Black patients treated with immunotherapy had a 15% lower risk of death from non–small cell lung cancer than non-Hispanic white patients.
Pertuzumab is indicated for use in combination with trastuzumab and docetaxel for treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Hodgkin lymphoma is most common in people between 20 and 40 years of age and those over 55 years of age.
Results of studies show about 61% of individuals with PV experienced a complete hematological response.

Pembrolizumab demonstrated a statistically significant improvement in disease-free survival, reducing the risk of disease recurrence or death by 32% compared to placebo.
Clinical trial findings show that the 2-year overall survival rate for those who received this was 72%.
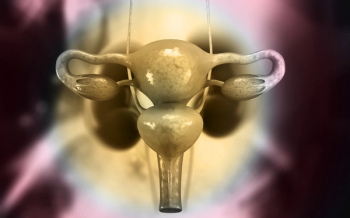
Treatment for endometrial cancer includes surgery, radiation therapy, chemotherapy, immunotherapy, and supportive care.

Pharmacists should keep an eye on the pipeline for autologous and allogeneic CAR T-cell therapies as well as antibody-specific treatments.

Atrial fibrillation was more common in those not treated with radiation (66.5%) or surgery (23.5%) as first-line treatment compared with 52.3% and 10.4%, respectively.
The panel also recommends considering risk factors for poor response, including uncontrolled disease, immunoparesis, the number of previous lines of therapy, and patient age.
In the IMPACT trial, women who were positive for high-risk HPV received a follow-up triage test to help determine whether their cervical cells were transforming to cervical pre-cancer.

The FDA initially approved regorafenib for chemorefractory metastatic colorectal cancer and unresectable gastrointestinal stromal tumor in 2012.
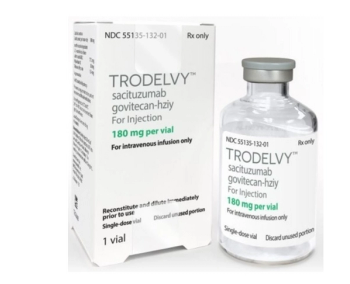
Sacituzumab govitecan-hziy is indicated for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received 2 or more prior systemic therapies, at least 1 of which for metastatic disease.
Bladder cancer most often begins in the cells that line the inside of the bladder.
Study results show that the type of vaccine patients received was a major factor in inducing an immune response, with those receiving mRNA shots faring better.

With so many approved regimens and common use of oral agents in the treatment of multiple myeloma, oncology pharmacists are essential team members.
More medication options mean a greater chance for patients to survive and enjoy a better quality of life.
Risk factors for liver cancer include chronic infection with hepatitis B or hepatitis C virus, cirrhosis, diabetes, and non-alcoholic fatty liver disease.
Study results show no differences in the rate of neuropathic pain, surgical site infection, wound-related complications, or other complications between the 2 groups.
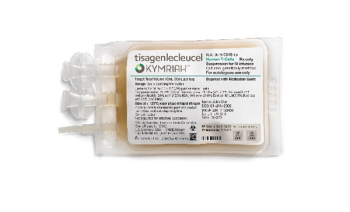
Kymriah is a CD19-directed genetically modified autologous T-cell immunotherapy.
Gaby Gabriel, MD, program director, interventional radiology residency and assistant program director, diagnostic radiology residency, University of Kentucky, discusses the implications of research into the treatment of liver metastases in patients with gastrointestinal NETs.

No new safety concerns were encountered, which the investigators said support the frontline use of daratumumab plus lenalidomide and dexamethasone for patients with multiple myeloma who are ineligible for transplantation.
Regularly testing before and during chemotherapy can help physicians determine early on if treatment is working.