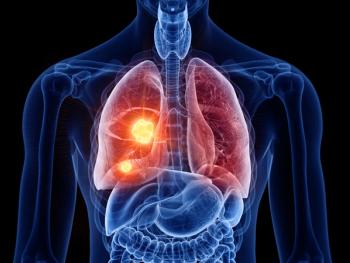
The FDA also granted traditional approval to amivantamab-vmjw for adults whose disease has progressed on or after platinum-based chemotherapy.

The FDA also granted traditional approval to amivantamab-vmjw for adults whose disease has progressed on or after platinum-based chemotherapy.

Hypomyelination with atrophy of the basal ganglia and cerebellum is the most severe form of TUBB4A leukodystrophy that currently has no curative treatment.
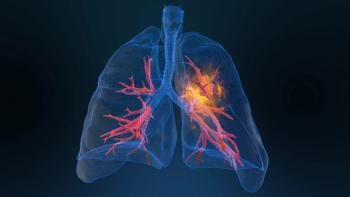
NVL-520 is a novel brain-penetrant ROS1-selective TKI being investigated for ROS1-positive metastatic non–small cell lung cancer in patients who have previously been treated with 2 or more ROS1 TKIs.
If approved, epcoritamab (DuoBody CD3xCD20; AbbVie, Genmab) would be the first and only subcutaneous bispecific antibody for treatment in this patient population.

The 3 bimekizumab indications include psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis.
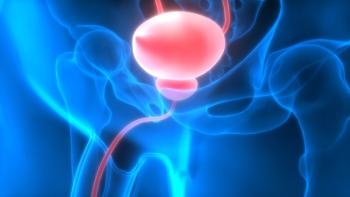
For more than 30 years, there has been little treatment advancement for NMIBC patients. Fortunately, a new development in the treatment of NMIBC has been approved by the FDA and is now available.

The data follow the FDA approval of omalizumab for the reduction of allergic reactions, including anaphylaxis, that can occur after exposure to 1 or more foods.
The new indication includes individuals with HIV who have suppressed viral loads with known or suspected M184V/I resistance.
The sNDA was based on results from the KRYSTAL-1 study, evaluating the drug alone or in combination with other anti-cancer therapies in those with advanced solid tumors who have KRASG12C mutations.

The withdrawal comes after clinical trial results failed to demonstrate the safety and efficacy of melphalan flufenamide when combined with dexamethasone.
Adalimumab-ryvk is the first high-concentration, citrate-free biosimilar to Humira that has been granted interchangeability status for the 40 mg/0.4 ml injection.

Currently, the safety and efficacy of linvoseltamab in adult patients with relapsed or refractory multiple myeloma is being compared to elotuzumab, pomalidomide, and dexamethasone in a phase 3 clinical trial.

Currently, ocifisertib is being evaluated in a phase 1b and 2 trial to confirm the safety and tolerability profiles in patients with acute myeloid leukemia.

Teclistamab was previously approved in October 2022 for the treatment of adult patients with relapsed or refractory multiple myeloma who received at least 4 prior therapies.
Datopotamab deruxtecan is an investigational TROP2 directed antibody drug conjugate that showed positive survival impact compared to chemotherapy.
If vorasidenib receives full approval, it would be a first-in-class target therapy to treat individuals with IDH-mutant gliomas.
Osimertinib was previously approved as a monotherapy, the first-in-line global standard of care, for non–small cell lung cancer indications.
This marks the first and only time that an individualized T cell therapy has been approved by the FDA for a solid tumor cancer.

This marks omalizumab’s fourth FDA-approved indication for allergic and inflammatory conditions, such as severe persistent allergic asthma, with its initial approval being in 2003.
Tigilanol tiglate is a novel, small molecule drug for the intratumoral treatment of solid tumors, inducing tumor cell death and stimulating immune response.
The approval of irinotecan liposome offers an improved first-line treatment option for individuals diagnosed with metastatic pancreatic adenocarcinoma.

Iloprost had also received Priority Review and Orphan Drug Designations for this indication, and FDA approval in 2004 for the treatment of pulmonary arterial hypertension.
Repotrectinib led to a durable anti-tumor response in patients with NTRK-positive locally advanced or metastatic solid tumors.

If approved, this would mark the first MDMA-assisted therapy and psychedelic-assisted therapy approved, calling for a reschedule of MDMA from Schedule I. A PDUFA was set for this summer.
The updated indication for the respiratory syncytial virus vaccine is for adults aged 50 to 59 with underlying conditions who are at an increased risk of contracting the virus.
Bepirovirsen is a triple action investigational antisense oligonucleotide and is being evaluated in the B-Well phase 3 clinical trial program for the treatment of chronic hepatitis B.

The FDA approval marks the first and only FDA-approved oral therapy for this patient population, and the drug is expected to be available by the end of February.
In a study, nipocalimab helped more than 50% of patients with high-risk of hemolytic disease of the fetus and newborn achieve a safe live birth.
The FDA assigned a Prescription Drug User Fee Act (PDUFA) goal date of October 8, 2024.

In November 2023, the FDA announced an investigation of CAR T-cell therapy based on several reported secondary T-cell malignancies.