Subcutaneous administration of atezolizumab and hyaluronidase-tqjs had similar efficacy to intravenous administration.

Subcutaneous administration of atezolizumab and hyaluronidase-tqjs had similar efficacy to intravenous administration.

Guselkumab (Tremfya; Johnson & Johnson) is the first and only approved fully human and dual-acting monoclonal antibody blocking interleukin-23 and binding to CD64 receptors.

FCS is a severe, rare genetic disease in which patients have extremely high triglyceride levels, typically above 880 mg/dL.
NS-050/NCNP-03 is being developed to aid individuals with confirmed gene mutations that are treatable with exon 50 skipping therapy.
Full traditional approval follows accelerated approval of the medication last year.

Edaravone and dexborneol sublingual tablets are an innovative first drug to be designed for treatment of stroke and granted this designation.
Previously, the FDA granted fast track designation to ABD-147 (Abdera Therapeutics Inc) for extensive stage small cell lung cancer.
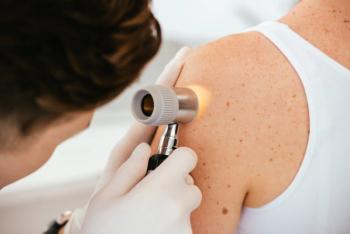
OBX-115 could offer further treatment options for individuals with advanced or metastatic melanoma by enhancing persistence, antitumor activity, and clinical safety of TIL cell therapy.
These companion diagnostics are poised to provide simpler and detailed insights into a patient’s genomic alternations, which could improve outcomes for patients with prostate cancer.

Bezisterim, an anti-inflammatory insulin sensitizer, will be authorized to proceed to a phase 2 clinical trial.
Mirdametinib is an oral, allosteric small molecule MEK inhibitor to treat pediatric patients with neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PN).

Previously, the device was indicated for the management of type 1 diabetes in patients aged 2 years and older.

With diagnostic tools, oncology pharmacists and other health care providers can help to identify treatments that have the best probability of working for a specific patient.
The indication is for adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma who were treated with at least 2 prior lines of therapy.

The proposed diagnostic agent has a long half-life, enables imaging the next day, and correlates to a longer shelf life in the pharmacy.
The acceptance will lead to vaccines that provide better protection against the KP.2 strain of SARS-CoV-2.
The FDA assigned the combination a Prescription Drug User Fee Act goal date of April 21, 2025.

The bispecific CAR T-cell therapy for treatment of multiple sclerosis is the first to target both CD19 and CD20.

The breakthrough therapy designation was granted based on ongoing data from the ARTEMIS-001 phase 1 open-label, multi-center trial.
Propofol has a history of chronic shortage due to supply chain constraints.
Vimseltinib (Deciphera Pharmaceuticals) has a Prescription Drug User Fee Act goal date of February 17, 2025.
The combination is the first and only multi-targeted chemotherapy-free regimen that demonstrated superiority compared with osimertinib for non–small cell lung cancer (NSCLC).

The highly potent, selective URAT1 inhibitor may help reduce serum uric acid levels and treat clinically visible tophi.

Fam-trastuzumab deruxtecan-nxki has received 4 breakthrough therapy designations, including the latest approval.

Currently, the drug will be studied in the SKYBRIDGE (NCT05652686) trial, a first-in-human, phase 1/2, open-label, dose-escalation and expansion study.

The OTC antibody test can detect current or past infection but should be followed by additional testing to confirm diagnosis.

The hemostatic gel is plant-based and designed to stop and control bleeding within seconds after applied to wound at the point of care.

Durvalumab is a human monoclonal antibody that targets and binds to PD-L1 to interrupt tumor immune-evasion tactics.
The supplemental biologics license application was granted based on results from the ADRIATIC phase 3 trial among individuals with limited stage small cell lung cancer.

The decision is based positive data from the Deltacel-01 clinical trial.