
In addition to oral administration, patients with partial-onset seizures can now take the antiseizure medication via a nasogastric tube if the tablet is crushed and mixed with water.

In addition to oral administration, patients with partial-onset seizures can now take the antiseizure medication via a nasogastric tube if the tablet is crushed and mixed with water.
Benralizumab was previously approved by the FDA in 2017 as an add-on maintenance therapy for patients 12 years and older.

The indication is for adult patients with multiple myeloma who are refractory to lenalidomide and have previously received at least 1 line of therapy.

The treatment is indicated for adolescents at least 12 years of age who weigh at least 25 kg without a previous treatment history of antiretroviral therapies.

This is the fifth indication for fam-trastuzumab deruxtecan-nxki (Enhertu; Daiichi Sankyo and AstraZeneca) approved by the FDA.
The approval expands the prior indication of idecabtagene vicleucel (Abecma; Bristol Myers Squibb), which will make the drug available to patients in earlier lines.

Bimekizumab-bkzx (Bimzelx; Union Chimique Belge) is a humanized monoclonal immunoglobulin G1 antibody that inhibits interleukin (IL)-17A and IL-17F.

New indications include Staphylococcus aureus bloodstream (SAB) infections, acute bacterial skin and skin structure infections, and community-acquired bacterial pneumonia.
The decision follows phase 3 trial results that demonstrated improved progression-free survival and confirmed the safety of Dato-DXd in patients with breast cancer.

Positive phase 3 trial results showed that danicopan was more effective than placebo when treating extravascular hemolysis in paroxysmal nocturnal hemoglobinuria.
The ODD comes after AP303 presented meaningful improvements in renal survival in an ADPKD and the completion of the first study that evaluated healthy human participants.
The new indication includes pediatric patients aged 6 years and older who weigh at least 25 kg and have compensated liver disease.

Vadadustat (Vafseo; Akebia Therapeutics Inc) is indicated for individuals with chronic kidney disease who have been receiving dialysis for at least 3 months.

The agency warns consumers against using these products, which are marketed for topical use to relieve pain associated with cosmetic procedures.
Sotatercept-csrk is the first approved activin signaling inhibitor therapy for pulmonary arterial hypertension, which represents a new class of therapy.
All 20 participants in a 12-month follow-up study attained acute success from ablation procedures.

The test scans whole blood samples from individual human donors for the 5 parasite species that can cause malaria in humans and detects Plasmodium RNA and DNA.

In December 2023, nipocalimab (Johnson & Johnson) was granted orphan drug designation for fetal neonatal alloimmune thrombocytopenia.

Previously, felzartamab received orphan drug designation and breakthrough therapy designation for the treatment of primary membranous nephropathy.
The orphan drug designation (ODD) comes after positive results from a phase 2 trial that showed cevidoplenib improved platelet counts by 63.6% and 40.9% in 2 different dose groups.
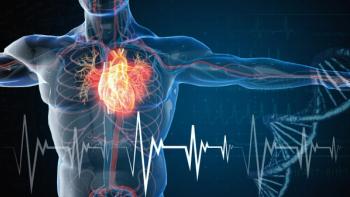
The combination tablet (Opsynvi; Johnson & Johnson) can also be used for individuals with pulmonary arterial hypertension who are being treated with stable doses of macitentan and tadalafil as separate tables.
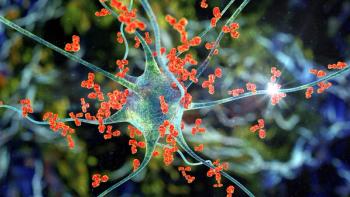
Zero relapses were reported among individuals that received ravulizumab-cwvz over the 73 weeks of treatment.
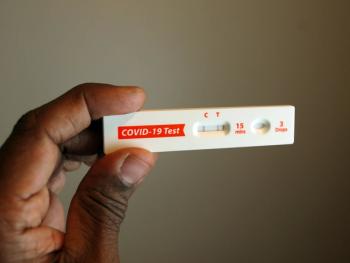
The indication is for adults and adolescents with moderate to severe immune compromise due to medical conditions or immunosuppressive medications and treatments.
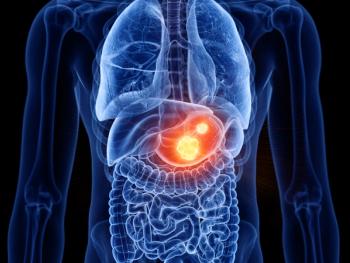
The orphan drug designation (ODD) is a result of positive findings from a phase 2 clinical trial which demonstrated a 100% clinical benefit rate in patients.
PT886 (Phanes Therapeutics) received fast track designation 2 years after receiving orphan drug designation by the FDA.
Bempedoic acid (Nexletol; Esperion) and bempedoic acid and ezetimibe (Nexlizet; Esperion) are the only LDL-C lowering non-statin drugs indicated for primary prevention patients.
The treatment is indicated for patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers.

The FDA recommends the dosage of givinostat (Duvyzat; Italfarmaco, ITF Therapeutics) should be based on the individual’s body weight and administered twice daily with food.

Rilpivirine (Edurant Ped; Johnson and Johnson) is indicated for the treatment of HIV in combination with other antiretroviral therapies in treatment-naïve pediatric patients.

The FDA has only approved aprocitentan (Tryvio; Idorsia) in the 12.5 mg dosage as the effects were similar between the 25 mg and 12.5 mg dosages.