
With this approval, Yimmugo is the first approved product from the Biotest Next Level production facility.

With this approval, Yimmugo is the first approved product from the Biotest Next Level production facility.
Repotrectinib has also shown significant efficacy in ROS1 fusion-positive non¬–small cell lung cancer (NSCLC), which was also studied in the TRIDENT-1 trial.

The autoinjector will provide another option for adults in addition to the prefilled syringe that is currently available.
The indication is for the improvement of glycemic control in pediatric patients aged 10 and older with type 2 diabetes and comes after positive results from the phase 3 T2NOW clinical trial.
Continuous glucose monitoring systems offer timely biofeedback for patients with type 2 diabetes.
Elafibranor (Iqirvo; GENFIT) is a first-in-class, once daily oral peroxisome proliferator-activated receptor for the treatment of primary biliary cholangitis.
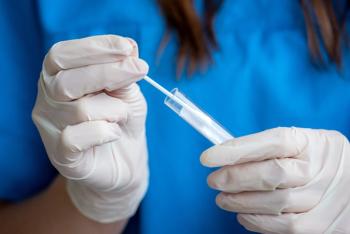
The 4-in-1 respiratory diagnostic test is either a single nasopharyngeal or anterior nasal swab that can confirm or rule out SARS-CoV-2, influenza A virus, influenza B virus, and respiratory syncytial virus with a single test.
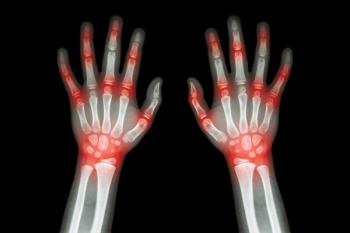
Polyarticular juvenile idiopathic arthritis is a form of arthritis that affects multiple joints at one time.

Osimertinib demonstrates improved progression-free survival for stage 3 epidermal growth factor receptor-mutated (EGFRm) lung cancer.
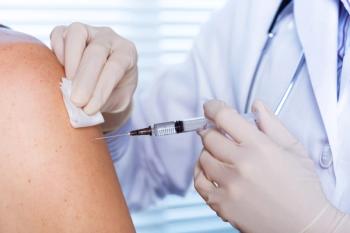
The expanded indication is for the prevention of respiratory syncytial virus-associated lower respiratory tract disease in adults aged 50 through 59 years who are at an increased risk.
The new approval increases the previous dosing for surface area treatment from 25 cm2 to up to 100 cm2.

Currently, the drug is being evaluated in a phase 1 clinical trial to determine the safety and efficacy in patients.

Imetelstat (Rytelo; Geron Corp) is a first-in-class telomerase inhibitor.
FKS518 (Fresenius Kabi) would be indicated for the treatment of osteoporosis in men and women, including glucocorticoid-induced osteoporosis and bone loss due to prostate or breast cancer.
Matthew A. Gubens, MD, MS, FASCO, highlights the importance of identifying genetic targets in metastatic non–small cell lung cancer (NSCLC), emphasizing the latest updates and strategies.

The priority review of inavolisib is for treatment of patients with advanced hormone receptor-positive, HER2-negative breast cancer with a PIK3CA mutation.

This is Moderna's second mRNA vaccine approval and the first mRNA vaccine approved for an indication other than COVID-19.

The treatment is indicated for adult patients with tardive dyskinesia and Huntington disease chorea, with tablets now available in 4 different doses.

If approved, zanidatamab will be the first HER2-targeted treatment indicated for individuals with this type of biliary tract cancer.

The treatment is the first liquid non-stimulant for attention deficit hyperactivity disorder to be approved in the United States.
Isatuximab is used in combination with bortezomib, lenalidomide, and dexamethanose.

Under the proposal, specific drugs would be prohibited

A lipoprotein (a) (Lp[a]) assay has been granted breakthrough device designation to expand identification of patients with elevated Lp(a) and genetic predispositions to cardiovascular disease.

Inavolisib was submitted to the FDA for review as a first-line treatment for advanced HR-positive, HER2-negative PIK3CA-mutated breast cancer.

The primary outcome was a 90-day incidence of mortality or liver transplant for individuals who received larsucosterol, compared to the placebo group.

Aflibercept-jbvf (Yesafili; Biocon Biologics) and aflibercept-yszy (Opuviz; Biogen, Samsung Bioepis) are the first interchangeable biosimilars to aflibercept (Eylea; Regeneron).
Belimumab (Benlysta; GSK) is a B-lymphocyte stimulator-specific inhibiting monoclonal antibody.
Tarlatamab is a first-in-class immunotherapy that binds to DLL3 tumor cells and CD3 T-cells, to kill DLL3-expressing SCLC.
The indication is for adult patients who have received at least 2 prior lines of systemic therapy and is based on the response rate and duration of response shown in a phase 2 trial.
SNB-101 is the first nanoparticle anticancer drug that was developed exceedingly insoluble SN-38 into polymer nanoparticles.