Antihemophilic factor (recombinant) Fc-VWF-XTEN fusion protein-ehtl was initally approved in February 2023 for adults and children with hemophilia A for prophylaxis and on-demand treatment to control bleeding.

Antihemophilic factor (recombinant) Fc-VWF-XTEN fusion protein-ehtl was initally approved in February 2023 for adults and children with hemophilia A for prophylaxis and on-demand treatment to control bleeding.

A provisional determination is also provided for the 40 mg/0.4 mL strength due to remaining interchangeability designation for another biosimilar to Humira.

This is the first approval of an EB virus-related mRNA therapeutic cancer vaccine and is a landmark achievement in future research on cancer treatment.

These developments highlight the dynamic landscape of health care innovation and the collaborative efforts driving progress in disease management and treatment.
The Rika system uses an individualized nomogram to help determine the plasma collection volume that is needed for each individual donor.

This is the first FDA advisory committee meeting that will review a potential new posttraumatic stress disorder treatment in 25 years.
The new administration method, which is co-formulated with rHuPH20, is usable in previous approved nivolumab indications for solid tumors in adult patients.
Currently, the treatment is being evaluated in the phase 3 CALYPSO study to further prove the safety and efficacy in patients with hypoparathyroidism.

This citrate-free and high-dose approval of the biosimilar Humira provides another option to treat patients who are living with various inflammatory diseases.
The 1-time treatment may be a better alternative for patients who don’t want to undergo frequent doses of standard of care intravenous factor IX infusions.

Tisotumab vedotin-tftv is the first antibody-drug conjugate to demonstrate positive overall survival data in patients with previously treated recurrent or metastatic cancer.

Trastuzumab is indicated for adjuvant breast cancer, metastatic breast cancer, and gastric cancer.
The acceptance is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials, as well as interim results from the phase 3 ADORING 3 open-label, long-term extension trial.

The indication is for patients aged 12 years of age and older, and the treatment is intended to increase the number of mature neutrophils and lymphocytes in patients.
Tovorafenib is the first systemic therapy to be approved for the treatment of pediatric patients who have low-grade glioma with BRAF rearrangements or fusions.
The approval is based on data from Study 5310, evaluating the pharmacokinetics, safety, and efficacy of Biktarvy (Gilead Sciences Inc) in pregnant individuals.

Dostarlimab plus chemotherapy is the only immuno-oncology-based therapy that showed statistically significant and clinically meaningful survival benefit in the overall patient population.

Naloxone hydrochloride (Amneal Pharmaceuticals Inc) is the generic equivalent to OTC Narcan (Emergent).

Pivmecillinam (Pivya; UTILITY therapeutics Ltd) tablets were approved for female adults with uncomplicated urinary tract infection caused by Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.

This approval makes lutetium Lu177 the first therapy to be approved for the treatment of gastroenteropancreatic neuroendocrine tumors in pediatric patients.
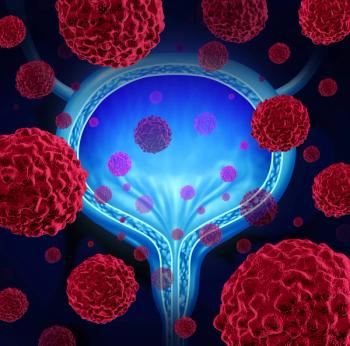
The approval was based on the QUILT-3.032 study, which included 77 adults with carcinoma in situ with or without papillary tumors after a transurethral resection.

The primary endpoint of the study was clinical remission at week 52 with vedolizumab
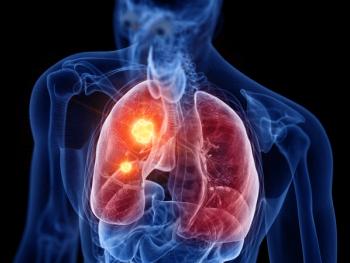
With this approval, alectinib is the first anaplastic lymphoma kinase inhibitor to be approved for patients with ALK-positive, early-stage non-small cell lung cancer.
The product is currently being investigated alongside TLX101 in the IPAX-Linz and IPAX-2 clinical trials, and have previously shown efficacy in the IPAX-1 trial.
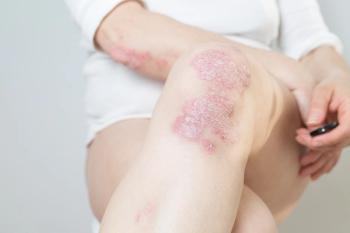
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.
If approved, the test could provide more timely and accurate diagnosis, hopefully mitigating the impact of Alzheimer disease (AD) on individuals and the community.

The 5-in-1 meningococcal ABCWY vaccine candidate has an assigned Prescription Drug User Fee Act action date of February 14, 2025.
Although the vaccine has not yet been tested in humans, it demonstrated 100% efficacy in protecting primates who were injected with human Marburg virus disease.
Treatment with LYT-200 is currently being assessed in a phase 1/2 adaptive design trial in advanced/metastatic solid tumors and in a phase 1b clinical trial.
The designation was granted based on overall survival data from an ongoing randomized phase 2 clinical trial.