A variety of methods can be used to create an individualized approach to removing barriers for patients with cancer.

A variety of methods can be used to create an individualized approach to removing barriers for patients with cancer.

The pipeline for biosimilar products in the United States includes at least 26 candidates in phase 3 trials for 13 reference therapies.

The DARE-19 clinical trial explores the use of SGLT2 inhibitors for patients with hypertension, cardiovascular disease, heart failure, type 2 diabetes, or chronic kidney disease, and COVID-19.

Novel treatments and recent data about new drugs were presented at the first conference of Advanced Topics for Oncology Pharmacy Professionals (ATOPP).
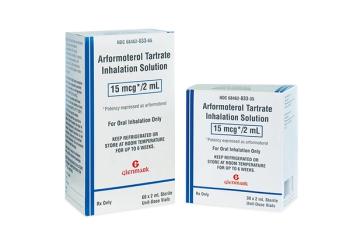
New drug products from Glenmark Pharmaceuticals and Cipla Limited are therapeutic equivalents to Sunovion Pharmaceuticals’ Brovana Inhalation Solution, 15 mcg/2 mL.

A 300 mg dose is currently available with a prescription.

The study examining venetoclax and obinutuzumab is in progress and currently recruiting patients who are newly diagnosed with asymptomatic, high-risk CLL.
The collaborative effort by pharmacies, which include Walgreens, CVS, and Albertsons Company stores, is part of the National Month of Action for COVID-19 vaccinations.
Data were presented at the virtual 2021 American Society of Clinical Oncology (ASCO) Annual Conference.

Analysis from the phase 3 ANDROMEDA study also shows doubling rates of organ response with no new safety signals for patients taking daratumumab and hyaluronidase-fihj.

Semaglutide (Wegovy) injection is indicated for use with a reduced calorie meal plan and increased physical activity to help adults with obesity or overweight, who also have weight-related medical problems, lose weight and maintain weight loss.

Results from the phase 3 TITAN study shows a combination therapy that includes apalutamide may achieve survival benefit without significant burden of adverse effects.
Notification letters were sent Monday to manufacturers found to violate 340B pricing after a review of the manufacturers’ actions and an analysis of complaints received from covered entities.

This product is a higher dosage of naloxone hydrochloride than the 2 mg and 4 mg dosage products previously approved by the FDA.
Oncology experts recommend patients schedule overdue screenings, as pandemic restrictions ease.
Selection of any BTK inhibitors for mantle cell lymphoma should consider factors such as cardiovascular or bleeding risks, predisposition for gastrointestinal adverse effects, and potential medication nonadherence.

Lakesha M. Butler, PharmD, BCPS, diversity and inclusion coordinator and clinical professor at Southern Illinois University Edwardsville, discussed causes of health inequity and how pharmacists can prevent and mitigate them to improve patient outcomes.

Common factors that contributed to reported increased dissatisfaction at work include role conflict, quantity of work, workflow disruptions, organizational culture, and leadership support.

Results presented at the 2021 APhA Annual Meeting and Exposition cite advertising and marketing difficulties as main barrier to success of point-of-care service.
Presentation provides an overview of recent FDA approvals for therapies utilized in patients with various cancer types.
The recommendations focus on dilution errors, vaccine and monoclonal antibody mix-ups, waste of doses, administering immunizations to the wrong age group, errors with scheduling the second dose, and allergic reactions.
Moderna, Inc has also announced temporary authorization of its COVID-19 vaccine in Switzerland.

In the past week, Moderna and AstraZeneca have collectively announced multiple authorizations for use of their respective vaccines.

This agreement brings the total number to be delivered to the United States to 200 million.
The ACIP advises the CDC on the populations and circumstances for which vaccines should be used.
Moderna’s COVID-19 vaccine (mRNA-1273) has been granted Emergency Use Authorization (EUA) by the FDA, making it the second vaccine to be issued an EUA for combating the virus.

If granted an EUA by the FDA, mRNA-1273 would become the second COVID-19 vaccine to receive the designation.
The specialty drug pipeline is coming off a strong year, and the oncology pipeline has a wealth of potential oncolytics.
The data presented at ASH 2020 build on results previously observed in the pivotal HAVEN clinical trial among adults, adolescents, and children with hemophilia A, with and without factor VIII inhibitors.

According to investigators, most medications should be stored at room temperature, between 68 and 77 degrees Fahrenheit.
Published: June 10th 2021 | Updated:
Published: April 15th 2021 | Updated:

Published: July 15th 2021 | Updated:
Published: April 14th 2021 | Updated:

Published: April 14th 2021 | Updated:

Published: October 16th 2019 | Updated: