
Faricimab-svoa targets and inhibits 2 disease pathways that drive diabetic macular edema and neovascular or “wet” age-related macular degeneration.

Faricimab-svoa targets and inhibits 2 disease pathways that drive diabetic macular edema and neovascular or “wet” age-related macular degeneration.
Ten quiz questions to assess your knowledge on common symptoms and treatments for glaucoma.

Data from 4 analyses show that individuals treated with the drug achieved non-inferior vision gains compared with aflibercept.

Lifitegrast ophthalmic solution (Xiidra) 5% is indicated for the treatment of the signs and symptoms of dry eye disease.
Cequa ophthalmic solution is indicated to increase tear production in patients with dry eyes.
Brimonidine ophthalmic solution (Alphagan P) is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Combigan is comprised of 2 components that reduce elevated intraocular pressure, whether or not associated with glaucoma.
If glaucoma is recognized early, vision loss can be slowed or prevented.
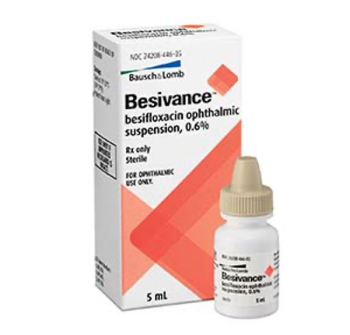
Besifloxacin ophthalmic suspension (Besivance) is indicated for the treatment of bacterial conjunctivitis.

But gene therapy may be the next big change; researchers recently identified a calcium modulator that protects the optic nerve in mice.
Pharmacists can play a key role in providing education and guidance to patients with glaucoma.

Fuchs' dystrophy causes fluid build up in the cornea, resulting in swelling.

Despite demonstrating numerous benefits, semaglutide has also been linked to an increased incidence of diabetic retinopathy, which was initially reported in the SUSTAIN-6 trial.

Blink Tears Lubricating Eye Drops maximizes hydration and restores tears’ natural salt balance.
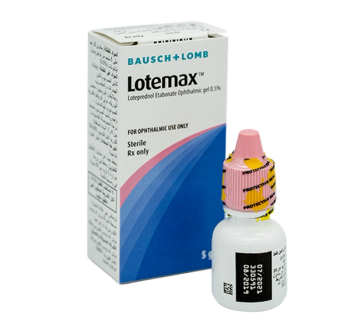
Lotemax treats post-operative inflammation and pain following ocular surgery.

AzaSite is indicated for the treatment of bacterial conjunctivitis caused by CDC coryneform group G, Haemophilus influenzae, Staphylococcus aureus, Streptococcus mitis group, and Streptococcus pneumoniae.

Farsightedness usually is present at birth and tends to run in families.
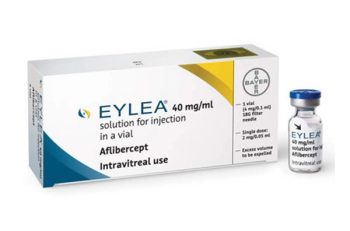
Aflibercept is indicated for the treatment of patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema, and diabetic retinopathy.

According to a press release from AbbVie, Vuity is the first and only FDA-approved eye drop to treat this condition, which affects nearly half of the US adult population.

According to the investigators, Susvimo is the first and only FDA-approved treatment for wet AMD that offers as few as 2 treatments per year.
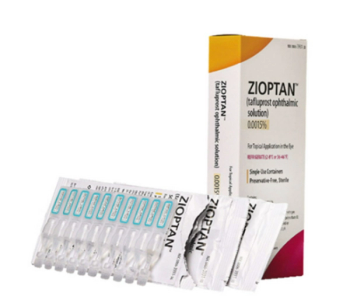
Tafluprost acid, a prostaglandin analog, is a selective FP prostanoid receptor agonist that is believed to reduce intraocular pressure by increasing uveoscleral outflow.

As the first nasal spray for dry eye disease, varenicline solution nasal spray could be especially helpful for patients who have difficulty administering eye drops.

According to GenSight, GS030 combines AAV2-based gene therapy with optogenetics to treat RP.

According to Ocular Therapeutix, Dextenza is now the first FDA-approved, physician-administered intracanalicular insert capable of delivering a preservative-free drug for the treatment of ocular itching associated with allergic conjunctivitis with a single administration for up to 30 days.

Doctors and pharmacists discuss OTC and prescription treatment options and use during webcast.