Eye Care
Latest News
Latest Videos
CME Content
More News

Switching from aflibercept (Eylea; Regeneron Pharmaceuticals) to biosimilar ranibizumab (Lucentis; Genentech) for neovascular age-related macular degeneration could be more cost effective for patients.

Prevalence of dry eye is increasing drastically, especially in younger individuals, because of the current lifestyle and increased technology use.

Following the previous FDA approval of faricimab-svoa to treat some of the leading causes of vision loss, the Administration has approved a single-dose prefilled syringe of the drug which could lead to safer and more efficient administration while easing patient burden.
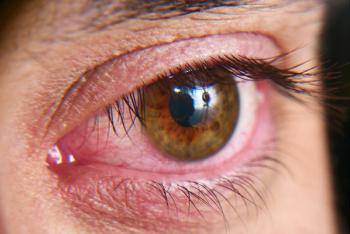
Caution Patients Against the Use of Redness-Reducing Eye Drops.

Aflibercept-jbvf (Yesafili; Biocon Biologics) and aflibercept-yszy (Opuviz; Biogen, Samsung Bioepis) are the first interchangeable biosimilars to aflibercept (Eylea; Regeneron).

In a review, investigators focused on ranibizumab (Lucentis; Genentech), aflibercept (Eylea; Regeneron), and bevacizumab (Avastin; Genentech) as growth opportunities in the biosimilar space.


Cardinal Health released its 2024 biosimilars report, including research and in-depth analyses on legislative development, payer landscapes, and new treatments in the pipeline.
Switching from adalimumab originator to SB5 did not cause clinically significant differences in treatment efficacy and safety for patients with noninfectious uveitis.

Teneo Excimer Laser Platform is a new laser assistance in situ keratomileusis for vision correction surgery for those who have myopia and myopic astigmatism.
Perfluorohexyloctane is the first and only treatment that targets tear evaporation to provide relief for dry eye disease
Preclinical studies and clinical trials have shown that both corticosteroids and VEGF inhibitors can be delivered as nanoparticles.

Faricimab is the first and only bispecific antibody granted FDA approval for treatment of the eye.

Pilocarpine improves near visual acuity by pupil modulation, leading to an increased ability to focus on nearby objects.

Teprotumumab (Tepezza) is a promising treatment for thyroid eye disease , although its precise mechanism of action remains incompletely characterized.
Cases address questions about how to relieve dry eyes.
The Mylight phase 3 trial confirmed that there was no clinically meaningful differences between aflibercept and its reference biologic, Eylea, for patients with wet macular degeneration.

Melphalan/hepatic delivery system (Hepzato Kit; Delcath Systems Inc) is currently the only liver-directed therapy approved by the FDA for the treatment of netastatic uveal melanoma and percutaneous hepatic perfusion.
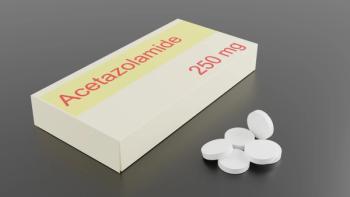
Idiopathic intracranial hypertension was previously considered a rare condition, however, its prevalence is increasing in parallel with obesity in patient populations.

SB15 had a comparable safety, pharmacokinetics, and immunogenicity treatment profile to AFL for age-related macular degeneration.
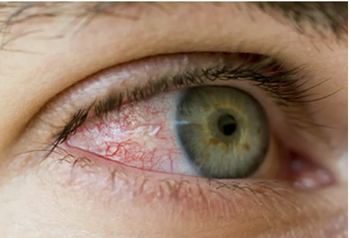
Pharmacists should learn the basics of this leading cause of vision loss among adults worldwide.

The approval marks the first and only FDA-approved treatment for dry eye disease that directly targets tear evaporation.

Data from the aflibercept 8 mg clinical trial program will be presented at the Association for Research in Vision and Ophthalmology (ARVO) 2023 Annual Meeting, April 23 to 27.
Eye drops come in many forms and for many indications, such as redness, itchiness, dryness, allergies, dilating drops, glaucoma drops, lubricants, numbing agents, antibiotics, and to lower pressure.

Aflibercept injection (8 mg) in a 12-week dosing regimen was found non inferior in vision gains compared to 8-week dosing regimen with 2 mg.