
Teprotumumab is the first fully human IgG1 monoclonal antibody approved by the FDA for the treatment of thyroid eye disease at its source not just reducing the symptoms.

Teprotumumab is the first fully human IgG1 monoclonal antibody approved by the FDA for the treatment of thyroid eye disease at its source not just reducing the symptoms.
Monkeypox may be transmitted through conjunctivitis, according to a new study.

Aflibercept 8 mg demonstrated non-inferiority in vision gains in both the 12- and 16-week dosing regimens.

Iheezo is a sterile, single use ophthalmic gel preparation.

Omidenepag isopropyl ophthalmic solution (Omlonti) eye drops are indicated to reduce elevated intraocular pressure in patients with primary open-angle glaucoma or ocular hypertension.

Researchers will submit a new drug application to the FDA after a phase 3 trial found that iDose TR slow- and fast-release models met their primary endpoint in patients with glaucoma.
Pharmacists should ascertain whether self-treatment is appropriate and suggest medical care if it isn’t.
Study suggests gene therapy may effectively treat young children who were born colorblind via pathways connecting the brain and the retina.
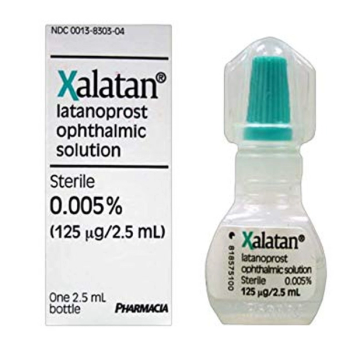
Latanoprost (Xalatan) Sterile Ophthalmic Solution is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.

Study is the first to use an artificial intelligence algorithm to break down visual field loss in new-onset glaucoma cases among population groups in the United States.
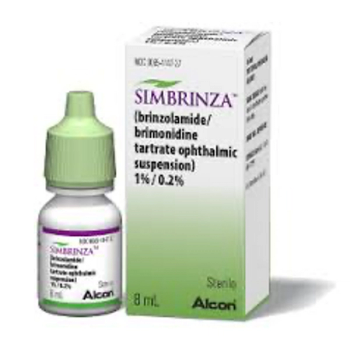
Brinzolamide/brimonidine tartrate (Simbrinza) is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.

Ranibizumab-eqrn is the only biosimilar approved for all 5 Lucentis indications.

Thera Tears helps with the symptoms of dry eyes.

Findings suggest potential screening and disease detection opportunities.

Treatment with faricimab-svoa (Vabysmo) may improve vision with fewer injections and treatment sessions for patients with wet age-related macular degeneration compared to other treatments.

If approved, the 16-week dosing regimen could offer certain individuals a potentially longer treatment interval and physicians with greater flexibility to individualize treatment.
Olopatadine stabilizes ocular mast cells to prevent histamine release by allergens.
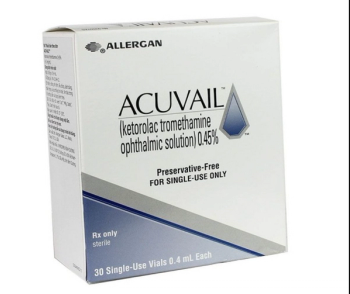
Acuvail ophthalmic solution is a nonsteroidal, anti-inflammatory indicated for the treatment of pain and inflammation following cataract surgery.

In clinical trials, Xiidra was found to improve eye dryness scores significantly compared with placebo.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the future of pharmacies as more cell and gene therapy products become available to patients.

Pilocarpine (Vuity) is a cholinergic muscarinic receptor agonist indicated for the treatment of presbyopia in adults.

PinkEye Relief are sterile eye drops that temporarily relieve burning, swelling, and crusting.

Brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% is a generic equivalent to AbbVie’s Combigan, which reduces eye pressure in patients with ocular hypertension.

The eye drop treats age-related blurry near vision in adults and is FDA-approved for once-daily administration.

The FDA has approved pilocarpine hydrochloride ophthalmic solution 1.25% (Vuity, Allergan, an AbbVie company) to treat presbyopia in adults.1