
Ali Dehghani, DO, highlights that shingles vaccination not only prevents rash but is also associated with lower risks of major cardiovascular events, dementia, and mortality, underscoring pharmacists’ role in patient counseling and preventive care.

Luke Halpern is an assistant editor with Pharmacy Times. Luke wrote for Pharmacy Times in the summer of 2023, and assumed a full-time role in June 2024. His work has been featured in Pharmacy Times and the American Journal of Managed Care. He graduated from the University of Massachusetts, Amherst in May 2024.

Ali Dehghani, DO, highlights that shingles vaccination not only prevents rash but is also associated with lower risks of major cardiovascular events, dementia, and mortality, underscoring pharmacists’ role in patient counseling and preventive care.

Nina Masters, PhD, MPH, explains how early adherence to pediatric vaccines strongly predicts timely measles-mumps-rubella (MMR) vaccination.

The epcoritamab, rituximab, and lenalidomide regimen provides a chemo-free, outpatient option for relapsed or refractory follicular lymphoma (FL), offering high response rates, improved quality of life, and expanding pharmacists’ roles in patient management.

Food insecurity and social instability significantly increase long COVID risk in children, highlighting the need for targeted public health interventions.

Ben Long, MD; and Weston Blakeslee, PhD, discuss how personalized text reminders significantly improve prescription adherence in patients with heart failure and the critical role pharmacists play in reinforcing engagement.

Jeffery A. Goad, PharmD, MPH, stresses that consistent, evidence-based communication from pharmacists and other health care professionals is essential to maintaining public trust and preventing declines in childhood vaccination.

Recombinant zoster vaccination against shingles was found to significantly reduce the risk of heart disease and dementia, even after a prior case of shingles.
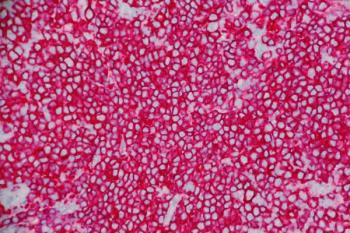
Zahra Mahmoudjafari explains how the epcoritamab, rituximab, and lenalidomide regimen delivers durable responses in relapsed or refractory follicular lymphoma while expanding the pharmacist’s role in dosing coordination and toxicity management.

Jeffery A. Goad, PharmD, MPH, explains how the shift toward shared clinical decision-making for pediatric vaccines may complicate counseling, reduce vaccine uptake, and increase public health risks.

Subcutaneous copper histidinate injections deliver copper in a form that bypasses the genetic defect caused by Menkes disease.

Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, explains how the epcoritamab, rituximab, and lenalidomide regimen delivers durable responses in relapsed or refractory follicular lymphoma while expanding the pharmacist’s role in dosing coordination and toxicity management.
Study author Nina B. Masters, PhD, MPH, highlighted an increase since the pandemic in the rates of patients missing their dose of measles, mumps, and rubella (MMR) vaccination at age 2 years.

Jeffery A. Goad, PharmD, MPH, explains how recent HHS policy changes reorganizing the childhood immunization schedule could complicate routine vaccination practices and increase the risk of gaps in pediatric disease prevention.

Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, highlights the EPCORE FL-1 trial, which showed that adding epcoritamab to rituximab and lenalidomide improved response rates and reduced progression risk in patients with relapsed or refractory follicular lymphoma.

The vouchers are intended to speed up review times for drugs that could fill key unmet needs for Americans.

Patients with type 2 diabetes (T2D) who initiated statins experienced reductions in major cardiovascular disease (CVD) and mortality across a spectrum of predicted 10-year risk.
Intravenous laser irradiation of blood shows promise in lowering triglycerides and improving lipid profiles in patients with dyslipidemia.
The complete response letter (CRL) was issued following regulatory updates provided to Sanofi from the FDA.

Tradipitant, a novel NK-1 receptor antagonist, was proven effective at reducing the incidence and severity of nausea and vomiting in individuals with motion sickness.

High levels of C-reactive protein in the blood and lower heart rate variability were linked to cardiovascular events in patients with depression, anxiety, or both.

Six months following pneumococcal vaccination, over 70% of patients lost their protective titers, suggesting the need for more optimal vaccination strategies.
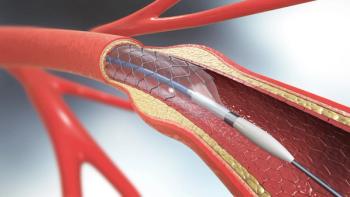
In patients with ST-segment elevation myocardial infarction, lowering low-density lipoprotein cholesterol to optimal levels prevented neoatherosclerosis, a complication following stent implantation.

A series of workplace factors were found to increase the risk of long COVID, including close contact with colleagues and commuting through public transportation.

The novel agent has demonstrated efficacy in symptomatic and less severe patients with obstructive hypertrophic cardiomyopathy in thorough phase 3 clinical trials.
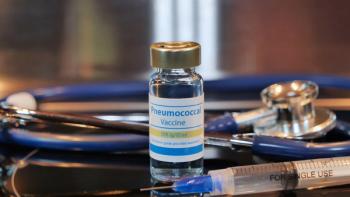
Serotypes included in the pneumococcal 21-valent conjugate vaccine (PCV21) cause outsized disease and economic burden in Norwegian patients with pneumococcal disease.
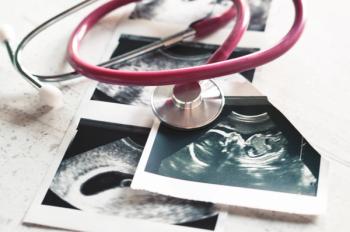
COVID-19 vaccination significantly lowers severe illness and preterm birth risks for pregnant individuals.
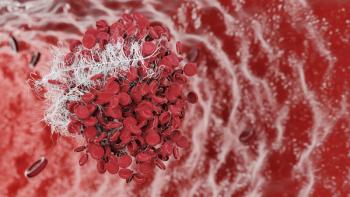
FDA approves Fesilty, a new fibrinogen concentrate, offering hope for effective treatment of congenital fibrinogen deficiency and acute bleeding episodes.

Previously approved for patients 12 years and older, berotralstat now gives younger patients a treatment option to manage sudden hereditary angioedema attacks.

The FDA has approved etripamil as the first and only self-administered nasal spray for adults with paroxysmal supraventricular tachycardia (PSVT), offering a rapid, effective treatment that can be used outside of health care settings.
New findings highlight the need for pneumococcal booster vaccinations in children with sickle cell disease to maintain immunity and prevent serious complications.