Hematology
Latest News
Latest Videos

CME Content
More News
Individuals with very low- to intermediate-risk myelodysplastic syndromes treated with luspatercept (Reblozyl; Bristol Myers Squibb) achieved improvements in hemoglobulin levels.
After allogeneic stem cell transplantation overall survival and relapse-free survival rates in patients with relapsed or refractory acute myeloid leukemia were 52% and 47%, respectively.

Imetelstat (Rytelo; Geron Corp) is a first-in-class telomerase inhibitor.
According to investigators, the primary and key secondary end points were met during the 52-week placebo-controlled period of the trial.
Asciminib demonstrated stronger efficacy, safety, and tolerability, as well as less adverse events in patients compared with those who received investigator-selected standard-of-care tyrosine kinase inhibitors.
Lisocabtagene maraleucel (Breyanzi; Bristol Myers Squibb) is a CD19-directed CAR T-cell therapy that is delivered as a one-time infusion.
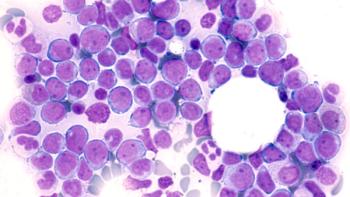
Researchers observed that patients aged 80 years and older with acute myeloid leukemia who were treated with venetoclax and a hypomethylating agent had prolonged overall survival.
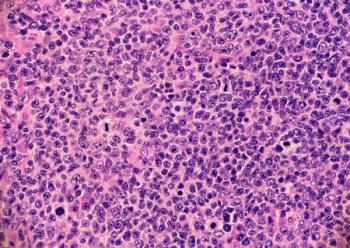
The indication is for adult patients who have received at least 2 prior lines of systemic therapy and is based on the response rate and duration of response shown in a phase 2 trial.

FDA approves groundbreaking gene therapies for sickle cell disease, revolutionizing treatment.

These developments highlight the dynamic landscape of health care innovation and the collaborative efforts driving progress in disease management and treatment.
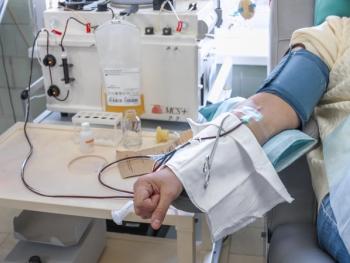
The Rika system uses an individualized nomogram to help determine the plasma collection volume that is needed for each individual donor.
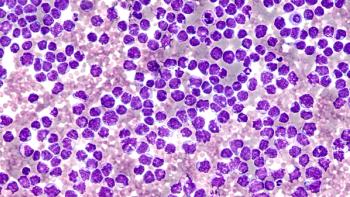
Compared with placebo, acalabrutinib plus standard of care chemoimmunotherapy demonstrated better progression-free survival in patients with mantle cell lymphoma.

Despite the increased survival rates in all 4 evaluated racial groups, both adult and pediatric non-Hispanic African American patients continued to have worse outcomes.

Advances, challenges, and promising innovations emerge.

Previously, glofitamab had received an accelerated approval for patients with relapsed or refractory diffuse large B-cell lymphoma who received 2 or more prior lines of systemic therapy.
This review discusses the prevalence, mechanisms of development, and evolving treatment landscape in chronic lymphocytic leukemia.
In this NCCN session, James M. Foran, MD, described the different FLT3 inhibitors that are available for FLT3-mutated AML, as well as considerations for the future.
The antibody drug conjugate was used in combination with the anti-cancer drug SG3249, and demonstrated efficacy in mouse models after 1 week.
The results demonstrated similar overall survival between patients with chronic lymphocytic leukemia who discontinued specialized follow-ups versus those who continued.

There are unique complexities when implementing bispecific antibodies, including site of care considerations, monitoring, and management of adverse events.
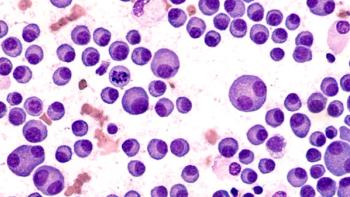
Unlike conventional monoclonal antibodies, BsAbs possess dual binding domains that target 2 distinct antigens simultaneously.
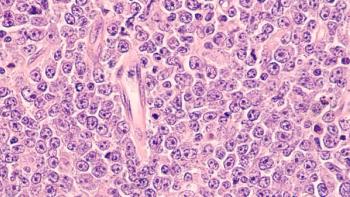
The mutation impacts BAF proteins and can lead to progression of follicular lymphoma, according to the investigators.

Positive phase 3 trial results showed that danicopan was more effective than placebo when treating extravascular hemolysis in paroxysmal nocturnal hemoglobinuria.
The mutations, C594Y and P735R, were found in the GNE gene and may influence sialylation, a process that is crucial for brain development.

Vadadustat (Vafseo; Akebia Therapeutics Inc) is indicated for individuals with chronic kidney disease who have been receiving dialysis for at least 3 months.