Understanding causes, consequences proves vital to develop therapies that exploit vulnerabilities of the cancer cells.

Understanding causes, consequences proves vital to develop therapies that exploit vulnerabilities of the cancer cells.

Treatment with epcoritamab-bysp showed a 61% overall response rate and a 38% complete response rate in heavily pretreated patients with R/R DLBCL.
The panel corresponds to proteins that are abundant in treatment-resistant melanoma cells and may predict resistance to targeted treatment.
Pegfilgrastim-pbbk is a leukocyte growth factor developed to reduce the onset of infection in patients with non-myeloid malignancies administered myelosuppressive anticancer medications associated with a clinically significant rate of febrile neutropenia.

Precision medicine is rooted in the reality that the way patients respond to treatment is heavily affected by their individual genetic variability, their complex biology, and external factors.

Fran Gregory, PharmD, MBA, vice president of Emerging Therapies at Cardinal Health, discussed the role biosimilars are playing in the pharmaceutical market as well as in patient access.
Lili Wang, MD, PhD, discusses her research investigating how METTL3 regulates RNA splicing dysregulation and contributes to CLL growth.

With a growing expectation for individualized and personalized care, medically integrated pharmacies have a great opportunity to support patients and the care team.
Individualized treatments like cell and gene therapies are poised to scale rapidly over the next decade.

Josh Marsh, vice president and general manager of Sonexus Access and Patient Support at Cardinal Health, discussed how patient assistance programs are leveraging technology.

A daily low-dose form of vitamin D3 was more effective on cancer mortality than intake of a monthly high-dose form, especially for adults aged 70 years and older.
Study of cell exhaustion in immunotherapy-resistant tumors could significantly improve the benefits of cancer immunotherapy for patients with treatment resistant types of cancer.
US Preventive Services Task Force also called for more research into how to address screening and treatment disparities faced by racial and ethnic minorities.

Importantly, Sullivan said data show that adverse events in the home infusion environment are no higher than in any other setting.

Approximately 43 total specialty drugs come to the market each year, on average.
Findings may offer insight into new strategies to tackle skin cancer in patients already treated with a prior line of therapy.

Kimlin Tam Ashing, PhD, discusses her research assessing effective interventions in ethnic minority communities to increase colorectal cancer screenings.

Pharmacists, in particular, must be cautious and deliberate in using ChatGPT to ensure that its benefits are fully realized while minimizing risk and misinformation.
Alpelisib plus the antioxidant N-acetylcysteine sensitizes NF1 knockout cells to PI3Kα inhibition and reverts their glycolytic phenotype in patients with advanced forms of breast cancer.
The novel antibody-drug conjugate is the first to demonstrate overall survival benefits in patients who were already treated with therapy.
The therapy had a clinically meaningful response in patients with relapsed or refractory (R/R) follicular lymphoma and R/R mantle cell lymphoma.

Study examines association between breast cancer survival and neighborhood-level measures such as socioeconomic status.
Immature data suggest enzalutamide combination improves overall survival in patients with high-risk biochemical recurrence.
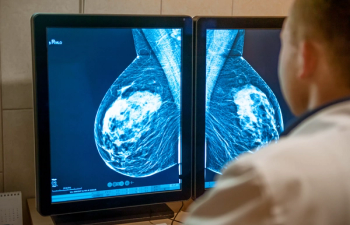
The system is considered effective at identifying residual breast cancer following surgical resection.
The treatment showed significant improvement in patients in patients who require frequent blood transfusions and are at risk of infection.
Relative risk of cancer-related death differed according to age of diagnosis.
Up to 80% of patients with hepatocellular carcinoma will experience disease recurrence.

Prediagnostic weight loss could be an important marker for colorectal cancer diagnosis.

Even before the Dobbs ruling overturned country-wide reproductive rights, the oncology field was already facing a dearth of maternal care access challenges.
Six weeks after infusion of GD2-CART01, 9 of 27 patients (33%) with relapsed or refractory high-risk neuroblastoma had a complete response or maintained a complete response.