
Earlier in June, Govindan led an educational session about genomic testing at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois.

Earlier in June, Govindan led an educational session about genomic testing at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois.
The folate alpha receptor (FRα) does have a toxicity profile, but unlike other chemotherapies, it does not severely compromise bone marrow and may improve ability to receive more therapies.

In-person visits to receive oral therapy, outpatient infusions, and inpatient infusion for the treatment of hematologic neoplasms declined in the early months of the COVID-19 pandemic, while telemedicine use increased slightly in the same period but remained low overall.
Achieving MRD flow cytometry negativity is crucial in monitoring and predicting survival outcomes.
Brentuximab vedotin with nivolumab and standard chemotherapy agents produced a nearly 100% overall response rate in patents with early-stage classical Hodgkin lymphoma.
A single infusion of ciltacabtagene autoleucel produced a lower risk of death and disease progression, as well as deep and durable responses, in heavily pretreated patients with relapsed/refractory multiple myeloma.
Providing this treatment for patients with cancer may be a smoother transition for oncology professionals than they might expect.
Expert provides numerous resources to learn more about the Centers for Medicare & Medicaid Services Enhancing Oncology Model.

Capivasertib combined with fulvestrant doubled progression-free survival in patients with recurrent or progressed advanced stage HR-positive HER2-negative breast cancer compared to placebo.
Additionally, data from the SCARLET trial presented at ASCO 2023 indicate the safety and efficacy of sotorasib in combination with platinum-based chemotherapy treating non¬–small cell lung cancer.

Even with technological advancements, provider involvement and oversight remain essential.

Denise Scarpelli, PharmD, MBA, discussed her presentation at the ASHP 2023 Summer Meeting.
Systemic treatment options have historically been limited to traditional chemotherapy agents.
Golidocitinib (DZD4205) is the first Janus kinase 1 inhibitor to be used for this aggressive and rare form of non-Hodgkin lymphoma.
Long-term data show that venetoclax combinations benefit progression free survival and overall survival in patients with or without previous treatment for chronic lymphocytic leukemia.

There are 8 million cancer survivors who may have limitations in their daily functional ability.
Investigators from the Barts Cancer Institute discovered that AMP-activated protein kinase is an essential sensor for tumor dissemination.

The meeting aims to bring together pharmacists to discuss solutions for responding to the rapidly evolving evidence and needs for effective implementation.

The combination also reduced the risk of developing distant metastasis or death by 65% in patients with high-risk stage 3/4 melanoma.
Trial results support 5.4 mg/kg of trastuzumab deruxtecan as the optimal dose in individuals with HER2+ metastatic colorectal cancer.
Until this study, adjuvant immunotherapy was standard of care for stage 3B melanoma but not stage 2, which has relatively similar survival rates and risk of recurrence.

Asking patients to “give us a month of [their] life” for autologous chimeric antigen receptor therapy may reduce relapse or recurrence for 4 years or more, according to expert.
Findings highlight the potential efficacy of combining 2 novel bispecific therapies, teclistamab-cqyv and talquetamab, which target distinct antigens on myeloma cells.
The phase 3 S1826 trial is the largest Hodgkin lymphoma study in NCTN history.
In data presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, amivantamab-vmjw plus lazertinib demonstrated promise as a first-line treatment for patients with advanced EGFR-mutated non-small cell lung cancer.
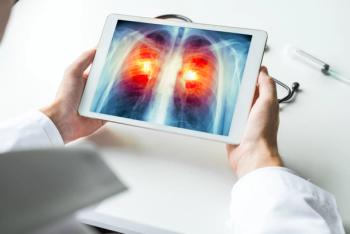
Nivolumab plus ipilimumab combination improved overall survival, with 21% of individuals with metastatic non–small cell lung cancer treated with the combination and 2 cycles of chemotherapy alive compared to 16% with chemotherapy alone.
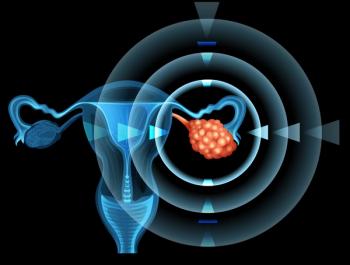
Positive findings in patients with advanced high-grade epithelial ovarian cancer were presented during an oral presentation at the 2023 American Society of Clinical Oncology Annual Meeting.
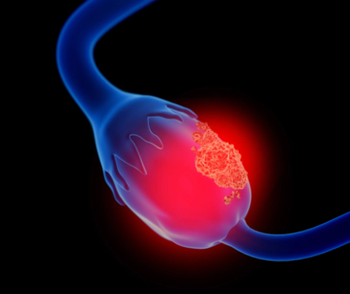
Expert suggests that mirvetuximab soravtansine-gynx, which received accelerated approval in the platinum-resistant ovarian cancer setting in 2022, is the most promising agent in more than a decade that targets folate receptor alpha.

Current standard of care gives 2 poor options to patients, neither of which cure grade 2 gliomas.
When physicians followed up, rates of screening improved for breast and cervical cancer by 50% and 250%, respectively, among eligible cancer survivors.