
It would also be beneficial to include a patient’s entire healthcare team in this management strategy, said an expert at the Hematology/Oncology Pharmacy Association (HOPA) Annual Conference 2023 in Phoenix, Arizona.

It would also be beneficial to include a patient’s entire healthcare team in this management strategy, said an expert at the Hematology/Oncology Pharmacy Association (HOPA) Annual Conference 2023 in Phoenix, Arizona.

Accelerated approvals for ibrutinib (Imbruvica) for patients with mantle cell lymphoma and with marginal zone lymphoma voluntarily withdrawn based on data that were insufficient to support conversion to full approval.
Consistent toxicity management and education reinforcement can improve the patient’s treatment management strategy.

The application is based on the PHAROS trial, which met its primary endpoint of objective response rate in patients with metastatic non–small cell lung cancer with a BRAF V600E mutation.
Study results show significant differences in pCR rates, but the differences were not subtype-specific.

Expert discusses the updated guidelines for treating diabetes at the American Pharmacists Association (APhA) 2023 Meeting & Exposition in Phoenix, Arizona.

Stephen Divers, MD, discussed the future of home infusion and drug development, and how both issues will be influential in the future of community pharmacy.

Improving the process of safe medication therapy management requires comfort, trust, and fostering a culture for safe communication.

Enfortumab vedotin-ejfv (Padcev, Astellas Pharma and Seagen Inc) with pembrolizumab (Keytruda, Merck) approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-containing chemotherapy.
Additionally, study results show a clinically meaningful overall survival trend in the overall population among individuals with primary advanced or recurrent disease.

Investigators suggest that funding mechanisms and interpersonal support systems are unsustainable for long-term care.
Oncology pharmacist discusses recent clinical trial data and what it could mean for patient outcomes.

A strong theme of recent conferences was the push for greater awareness of pharmacists' roles in patient advocacy and patient education.

Some common questions for oncology pharmacists are about concurrent use of complementary and alternative medicine supplements and natural products.

Clinical trial findings show that olaparib, given in the neoadjuvant setting, was feasible with 100% of participants able to receive the planned 2 cycles.

Factors (comorbidities, social factors) can largely affect the success of therapies in daily practice, despite findings observed in clinical trials.

And whatever specialty the student or resident decides on, it does not have to define the rest of their career.

Creating a safe environment for LGBTQ+ patients through the use of trauma informed care is critical to ensuring they feel comfortable returning to the clinic in the future.

There has been a historical lack of complete or appropriate data on sexual and gender minority groups, leading to difficulties in analyzing health outcomes in LGBTQ+ community.
Despite the potential for clinical benefit, triplet therapies have added complexities, monitoring needs, toxicities, and costs associated with additional pharmacologic therapy.
If patients with G6PD deficiency are exposed to risk factors, such as certain medications, environmental factors, or foods, it can lead to hemolysis.
Pharmacists have an important role in helping patients to identify treatment-related toxicities and manage symptoms to help the transition to the outpatient setting.
Expert notes significant advances in the treatment of multiple myeloma, large B cell lymphomas, and acute myeloid leukemia, during a presentation at HOPA 2023 Annual Conference.

The Hematology/Oncology Pharmacy Association (HOPA) created committees, inclusion statements, and diversity, equity, and inclusion (DEI) checkpoints to improve healthcare for underserved or historically less included populations.
Before tapering patients treated for cancer off of opioids, care providers should first establish what the goals of tapering are.
The data underscore the need for biomarker testing to identify patients who may be eligible for this therapy, according to physician from Memorial Sloan Kettering Cancer Center.

Certain populations have been found to have differing prognoses, such as African American patients, who have an earlier onset of disease with a higher likelihood of an aggressive tumor type and shorter survival.
Experts note that pharmacists are well positioned to play a key role in precision oncology and molecular tumor boards.
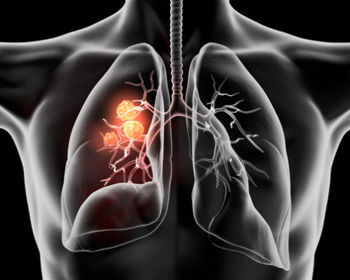
Historically, there are many hard-to-treat mutations of non-small cell lung cancer.

President (2022 to 2023) of the Hematology Oncology Pharmacists Association (HOPA) highlights the many ways that hematology/oncology pharmacists can collaborate with care teams to improve access to care at HOPA Annual Conference 2023 in Phoenix, Arizona from March 29 to April 1.