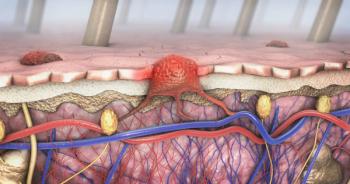
The microRNA member miR-204-5p inhibits apoptosis in melanoma cells treated with dacarbazine or when overexpressed.

The microRNA member miR-204-5p inhibits apoptosis in melanoma cells treated with dacarbazine or when overexpressed.
Race, ethnicity, years after diagnosis and mental and physical stress are among factors associated with unmet supportive care needs and a higher risk of hospitalization among patients with cancer.
Increases in biological aging based on epigenetic measures could be used to inform the future of survivorship care among patients with breast cancer.
Memory killer cells have been shown to respond to immunotherapy, which is normally administered as a complement to other cancer treatments.

This month, we have a special crossover episode between Pharmacy Focus: Oncology Edition and the Psychedelic Pharmacy podcast.
Approximately every 2.2 minutes, a new diagnosis of lung cancer is registered.
The approval was based on data from 2 studies of blinatumomab in adults and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia.

Treatment with lisocabtagene maraleucel led to strong and durable response rates with little serious adverse effects in patients with relapsed or refractory follicular lymphoma and mantle cell lymphoma.
Clinical trial results showed that the combination significantly improved radiographic progression-free survival compared with enzalutamide monotherapy for homologous recombination repair gene–mutated metastatic castration-resistant prostate cancer.

Approximately 50% of patients with refractory or relapsed disease achieved complete response following treatment with the Janus kinase 1 (JAK1) inhibitor.

In 2018, brentuximab vedotin was approved for advanced Hodgkin lymphoma based on an improvement in progression-free survival of 82.3% in combination with chemotherapy.
Trial results demonstrated that 58.5% of individuals receiving luspatercept-aamt (Reblozyl; Bristol Myers Squibb) achieved the primary endpoint of red blood cell transfusion independence of at least 12 weeks.

Pharmacists can help patients live a life of meaning and gratitude after cancer recovery.
The single institution study compared fixed dose capecitabine to standard dose capecitabine to compare efficacy and tolerability in metastatic breast cancer.
The benefit of ribociclib was seen as consistent against all of the stratification factors in the phase III study.
Heidi Finnes, PharmD, BCOP, FHOPA, addresses how the study data may impact the treatment of IDH1/2 mutating gliomas going forward.
Panelists discuss the potential impact of the study data presented at the ASCO 2023 Annual Meeting, which led to significant response from the field.

Andre Harvin, PharmD, MBA, discussed the use of immunotherapies in melanoma and broader challenges with access to oncology care in rural or underserved communities.
Ryan Haumschild, PharmD, MS, MBA, discussed his presentation at the Oncology Pharmacists Connect meeting, taking place June 15 through 17 in Austin, Texas.

The ASCO abstracts were on the TALAPRO-2 trial (abstract 5053) and PROpel trial (abstract 5012).

With immune checkpoint inhibitors in particular, patients are seeing greater results with fewer adverse effects.
For squamous cell carcinoma systemic therapy that is not concurrent with radiation therapy, preferred regimens are immune checkpoint inhibitors.
Both immunotherapies and targeted therapies are showing significant promise in these areas, offering patients new treatment options.
Andrea Iannucci, PharmD, BCOP, assistant chief pharmacy officer at UC Davis Health, discussed challenges and opportunities when implementing new treatments for breast cancer.
Heidi Finnes, PharmD, BCOP, FHOPA, director of clinical ambulatory pharmacy practice at Mayo Clinic, discussed updates in melanoma and the use of immunotherapies and targeted therapies.
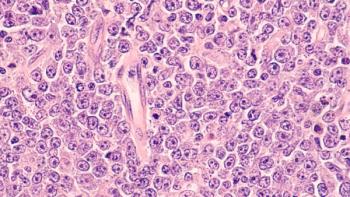
A single dose of axicabtagene ciloleucel in patients with large B-cell lymphoma led to significantly higher overall survival and progression-free survival rates, as well as improvements in quality of life and faster recovery in comparison to standard care therapy.
A plethora of targeted therapies, biomarker-driven therapies, and immunotherapies are now available.

Imposing the FDA on-label/off-label framework on administratively defined "children" resulted in a regulatory demand for pediatric studies that had no basis in clinical medicine, with the exception of the small group of preterm newborns.

Glofitamab targets CD3, a protein found on the surface of immune T cells in patients with relapsed or refractory diffuse large B-cell lymphoma, and CD20, a healthy or malignant protein that lines the surfaces of B cells.

Depression can affect the trajectory of treatment and associated outcomes.