Ten quiz questions to assess your knowledge on common symptoms and treatments for Clostridioides difficile.

Ten quiz questions to assess your knowledge on common symptoms and treatments for Clostridioides difficile.
VOR33 aims to protect an individual’s healthy cells from anti-CD33 therapies and replace standard care transplants.

New research has found that the relative cardiovascular safety of different types of androgen deprivation therapy (ADT) for prostate cancer remains unresolved after the PRONOUNCE trial was terminated early.
Managing transitions of care is vital to discontinue use of unnecessary drugs and minimize medication errors.

The results of a 5-year follow-up study comparing trastuzumab (Ontruzant) and reference medicine trastuzumab show comparable cardiac safety profiles and long-term efficacy.
The findings, published in the Journal of the American Heart Association, add to a list of cardiac risks experienced by patients with HIV.
These special immune system defense molecules help show which individuals are most at risk of needing intensive care and need to be monitored more closely, new study results show.
With CAR T-cell therapies showing promise for follicular lymphoma and marginal zone lymphoma, clinicians must weigh toxicity and tolerability almost as strongly as efficacy because patients can live years with these diseases

Lola Fashoyin-Aje, MD, MPH, associate director of Science & Policy Program to Address Disparities at the FDA’s Oncology Center of Excellence, discusses the importance of promoting inclusion of members of racial and ethnic minority groups in cancer drug trials.
The European Association of Neuro-Oncology and the European Society for Medical Oncology published recommendations related to parenchymal brain metastases.
Results from POSEIDON, a randomized open label, phase 3 trial, showed that patients administered 5 cycles of tremelimumab plus durvalumab and chemotherapy over 16 weeks experienced a 23% reduction in the risk of death compared with various chemotherapy options.

Study suggests potentially expanding the use of immunotherapies in the elderly, a population in whom these therapies may be under-prescribed.

The Pharmacy Quality Alliance developed the “Medication Access Framework for Quality Measurement” to address the social determinants of health that hinder patient medication access and contribute to poor health outcomes.

Six-point plan outlines strategies to increase national vaccination rates, keep schools open, increase testing and masking access, and to relieve health systems that are overrun with COVID-19 patients.
The efficacy of pirtobrutinib does not depend on prior therapy, reason for prior BTK inhibitor discontinuation, or C481 mutation status, according to the presentation.

Empagliflozin demonstrated a 21% relative risk reduction for the composite primary endpoint of cardiovascular death or hospitalization for heart failure, compared with placebo.
Prostate cancers grow slowly and rarely cause any health problems, so without regular screening, it is more difficult to identify any cancer in the prostate.
Ado-trastuzumab (Kadcyla, Genentech) was approved by the FDA in 2019 for the adjuvant treatment of patients with human epidermal growth factor receptor 2-positive early breast cancer.

Nearly 20% of patients with multiple myeloma have a form of the disease in which they make high quantities of a component of monoclonal proteins, which damages the kidneys.
The investigators said that even patients with asthma showed no statistically significant deterioration in lung function, although there was a trend toward slightly lower measurements for the amount of air they could exhale forcibly in 1 second.

Bleeding in the upper GI tract is among the most common bleeding complications following acute myocardial infarction, causing considerable suffering and increasing the risk of death.
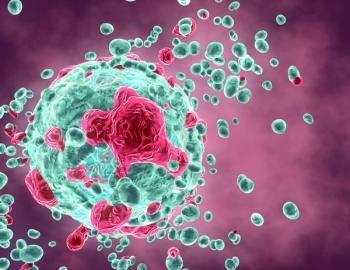
This drug class works by interfering with the ability of cancer cells to repair themselves after experiencing damage to their DNA, but how PARP inhibitors selectively kill cancer cells was previously unknown.
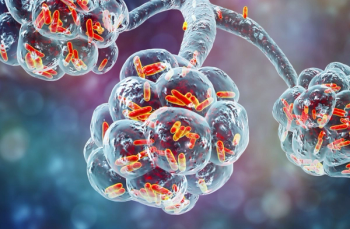
The results of a study show that with proper antibiotic treatments and therapy, clinical outcomes for those with community-acquired pneumonia do not differ.

Study results show that these personalized treatments adjust based on cerebral function through electric pulses.
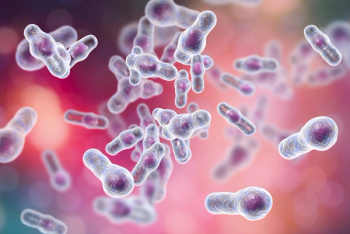
From an economic perspective, C. difficile has a staggering financial impact on the US health care system, as well on the patients infected.

Diabetic kidney disease develops in nearly 40% of patients with diabetes and serves as the leading cause of chronic kidney disease worldwide, according to the study.

As specialty drugs move through clinical trials and preparations for commercialization begin, significant attention is warranted to this process considering the massive investments that would have been already made in the product up to this point.

Harry Webster, RPh, a registered pharmacist at Walgreens and a member of the LGBTQIA+ community, discusses the impact of stigma associated with PrEP and HIV on patients seeking to treat or prevent HIV.

Michael Ganio, PharmD, MS, BCPS, FASHP, Senior Director of Pharmacy Practice and Quality at the American Society of Health-System Pharmacists, discussed the recent strains for hospitals due to the newest wave of COVID-19.

New evidence shows that moving to urban areas in the Midwest or either coast could increase the longevity of an individual’s life.