Although there are several conditions known to be risk factors for clostridium difficile, data on the link between malignancy and clostridium difficile are limited.

Although there are several conditions known to be risk factors for clostridium difficile, data on the link between malignancy and clostridium difficile are limited.
Enfortumab vedotin-ejfv (Seagen; Padcev) is a first-in-class antibody-drug conjugate approved for patients with previously treated locally advanced or metastatic urothelial cancer.
The prostate-specific membrane antigen-targeted positron emission tomography imaging technique will be part of the National Comprehensive Cancer Network guidelines beginning in 2022.
Although frontotemporal dementia is the leading cause of the disease for those under 65 years of age, far fewer people are familiar with it than with Alzheimer disease.
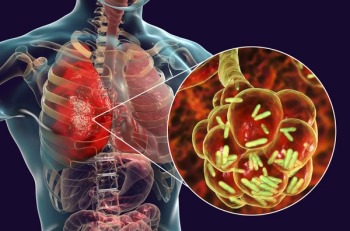
Study shows Lefamulin, omadacycline, ceftaroline, delafloxacin are beneficial in treating community-acquired pneumonia.
Ribociclib is an inhibitor of cyclin-dependent kinase 4 and 6 and causes tumor growth inhibition.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses how the treatment of advanced cutaneous T cell lymphoma has changed in recent years and whether there have been any advancements of note.

Andrea Silber, MD, said the COVID-19 pandemic has exacerbated disparities that already existed within oncology care, and she said the effects of these disparities on patients are very real and direct.

The overall response rate of ruxolitinib at week 24 was 49.7% compared with 25.6% for the best available therapy treatment for graft-versus-host-disease.

The COVID-19 booster doses are to be administered at least 6 months after completion of the primary 2-dose series and are the same formulation and dosage strength as the original doses.
Remdesivir demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19-related hospitalization or all-cause death by day 28.
Crizanlizumab is a humanized IgG2 kappa monoclonal antibody that binds to P-selectin and blocks interactions with its ligands.

ALS results in muscles that are reduced in size (atrophic), weak, and soft, or muscles that are stiff, tight, and spastic.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, provides an overview of key trials that have been conducted in the advanced cutaneous T cell lymphoma space in recent years.
Patients with triple-negative breast cancer who received sacituzumab govitecan had a median progression-free survival of 4.6 months compared to 2.3 months with chemotherapy.
Khalili and his colleagues have completed preclinical studies that have shown that EBT-101 can effectively excise HIV proviral DNA from the genomes of different cells and tissues, including HIV-infected human cells and cells and tissues of humanized mice.
Several studies have shown that a pharmacist’s presence on a sepsis team has a statistically significant impact on patient outcomes, with pharmacists taking on several roles during patient management.
Immune globulin subcutaneous (human), 20% solution (Cuvitru) is approved for primary immunodeficiency in patients 2 years of age and older.
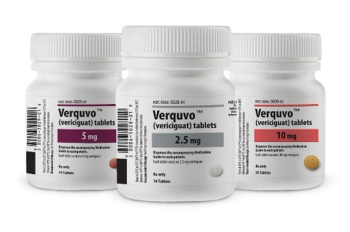
Verquvo is a soluble guanylate cyclase stimulator indicated to reduce the risk of cardiovascular death and heart failure.
Macrolide resistance varied by US region but was greater than 25% in most areas and 39.5% overall.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explained whether the current advanced cutaneous T cell lymphoma (CTCL) treatment strategies available are applicable for all patients with advanced CTCL and how patients are selected for treatment.
The results of 3 phase 3 trials showed that the drug offered greater proportions of individuals with the condition who met the endpoints of clinical remission and endoscopic response.

With the recent announcement of federal COVID-19 vaccine mandates, in addition to private businesses potentially requiring vaccines for employees, pharmacies and other health care facilities could have questions about legal issues surrounding these mandates.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, described what advanced cutaneous T cell lymphoma is in terms of classification and what current treatment approaches are available.

Trial results showed a 24% confirmed objective response rate with a median duration of response of 8.3 months among patients with cervical cancer who received tisotumab vedotin-tftv.
Relatlimab is the first LAG-3-blocking antibody to demonstrate a clinical benefit for patients with melanoma in phase 3 data.

Phase 3 trial results show that the drug reduced the risk of death or reoccurrence by 35% for individuals with high-risk disease compared with the placebo.

Nearly 94.1% of individuals treated with the drug in the DESTINY-Breast03 study were still alive at the 1-year mark, according to a statement from AstraZeneca.
Cisplatin is frequently an effective therapy for pediatric cancer, but it is also known to cause permanent hearing loss in some patients.

The results are the first from a pivotal trial of any COVID-19 vaccine in children under 12 years of age, with results in children under 5 years of age expected as soon as later this year.