
Washington State University study results show that slumber-deprived individuals had more difficulty reducing the emotions they felt when so instructed than those who slept normally.

Washington State University study results show that slumber-deprived individuals had more difficulty reducing the emotions they felt when so instructed than those who slept normally.

Abemaciclib (Verzenio) approved in combination with endocrine therapy for the adjuvant treatment of adult patients with HR–positive, HER2-negative, node-positive, early breast cancer at high risk of recurrence.

Adults 40 to 59 years of age should consult their clinician before taking aspirin, and adults 60 years of age and older should stop aspirin use for the prevention of cardiovascular disease.
Michael Frank, CEO of Revive Therapeutics, and Derrick Welsh, COO of Psilocin Pharma, a division of Revive Therapeutics, discuss clinical research investigating novel treatment targets for psilocybin, such as treatment-resistant depression in patients with cancer.

Pharmacy Times spoke with Dr. Jeffrey Akers, CEO of Pharmaceutical Services at Appalachian Regional Healthcare, about how integrated technology can empower pharmacists to prioritize patient care and safety and can help prevent harmful medication events from occurring.

Although October has long been celebrated as National Pharmacy Month, it is only in recent years that female pharmacists have been celebrated for their contributions to the profession and patient care.

The authors said stigmatization threatens societal cohesion during times of national upheaval, such as during a pandemic.

Jameshia Below, PharmD, assistant professor of pharmacy practice at the University of Louisiana Monroe College of Pharmacy, explains how oncologic emergencies present in patients, and how each type can be recognized.

Patients receiving sacituzumab govitecan also reported lower symptomatic impact of fatigue, pain, dyspnea, and insomnia.
People who inherit familial hypercholesterolemia from both parents usually develop symptoms in childhood.

Fluciclovine F 18 (Axumin), which is a radioactive diagnostic agent for injection that can be used to detect recurring prostate cancer.
The PROpel phase 3 trial met the primary endpoints of radiographic progressing-free for men with metastatic castration-resistant prostate when treated with Olaparib, combined with abiraterone.
The medication, which has been cleared as a treatment for the inflammatory disease of the throat, is under priority review by the agency.
There are 3 PARP inhibitors that are FDA approved in multiple settings of ovarian cancer.

The study aimed to examine the impact of masking and social distancing practices used to control the spread of COVID-19.

The majority of treatment-related adverse events (TRAEs) were grade 1-2 in severity, and no patients experienced dose-limiting toxicities during the 28 days following initial treatment.

Allostatic load is caused by a number of different stressors, including social isolation, poverty, and racism, many of which are commonly experienced by individuals belonging to racial and ethnic minorities, according to the investigators.
Types of seizure include focal seizures with impaired awareness, generalized seizures without loss of consciousness, and generalized seizures, such as absence seizures.

Winners were announced and celebrated at the Next-Generation Pharmacist® of the Year gala on Oct. 8 at Founders Hall in Charlotte, N.C.

The most common medication errors seen in LTC facilities include dispensing errors, delay in delivery, and expired inventory.

There is a real opportunity for technology to alleviate some of the administrative burdens pharmacists face so that they have more time to focus on patient care.
Samples for the test are isolated from nasopharyngeal swabs, anterior nasal swabs, and mid-turbinate swabs.

The investigators said that these cells, referred to as lymph node resident memory T cells, have been demonstrated to counteract the spread of melanoma in mice.

Avacopan (Tavneos, ChemoCentryx) met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks during a phase 3 trial in patients with severe active anti-neutrophil cytoplasmic autoantibody-associated vasculitis.
Cemiplimab is a recombinant human immunoglobulin G4 monoclonal antibody that binds to PD-1 and blocks its interaction with PD-L1 and PD-L2.
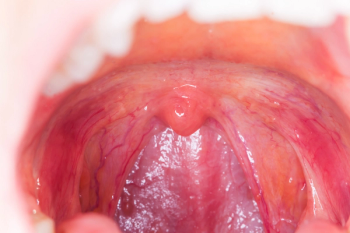
Tonsillitis most commonly affects children between preschool ages and the mid-teenage years.

As practices have gained experience in the post-COVID-19 environment, health care professionals have found innovative ways to work around many of the obstacles the pandemic presents.

Medication adherence, diet, and exercise are each critical components in caring for patients with a chronic illness, but each is difficult to be managed by physicians alone.
CAR-NK therapies are also shown to have fewer adverse reactions, and 25% of mice that were treated remained disease-free.