
Research shows that individuals who had either a severe or mild case of the virus had an increased risk of kidney damage and disease.

Research shows that individuals who had either a severe or mild case of the virus had an increased risk of kidney damage and disease.

Study shows the benefit of green and blue spaces for individuals with COPD living in urban communities.

As pharmacists were pulled into patient-facing roles, many hospitals were forced to scale back or make adjustments in medication compounding practices.
The guidance noted it is preferable for immunocompromised patients to receive their third dose in a health care delivery setting rather than a pharmacy or public vaccination clinic.

COVID-19 convalescent plasma administered within the first week of symptoms did not prevent disease progression in a high-risk group of outpatients.
Kidney disease is among the most common causes of both hyperkalemia and hypokalemia.

Following a phase 2 study, Moderna submitted initial data to the FDA for the approval of its COVID-19 booster shot that would be administered 6 months after an individual’s second dose.

Calls to poison control centers regarding the use of ivermectin for the prevention or treatment of COVID-19 have increased 5-fold since its baseline before the COVID-19 pandemic.

A study found individuals receiving probiotics had a decrease in C. difficile diagnosed in stool samples than those who did not receive probiotics.
Vaccinations are a huge success story in global health care and have helped eliminate smallpox and nearly eradicate polio.

The FDA has approved a second drug, zanubritinib, for the treatment of adult patients with Waldenström’s macroglobulinemia.

The study authors note that they cannot conclude that there is a causal relationship between the COVID-19 vaccines and any of the individual incidents of Bell palsy reviewed.

AZD7442 reduced the risk of developing symptomatic COVID-19 by 77% compared to placebo.
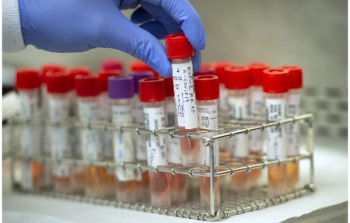
Lab values for sodium, potassium, chloride, magnesium, BUN, creatinine, glucose, and CO2.

Individuals with COVID-19 were more likely to be asymptomatic if they contracted the virus from a primary case that was also asymptomatic.

Atezolizumab was granted accelerated approval for the mTNBC indication in March 2019, making it the first immunotherapy agent to be approved in this setting.

The FDA's green light for the Alzheimer medication is worrisome, as clinical trials proved inconclusive.

Collaborative efforts between patients, physicians, pharmacists, and other essential personnel can further promote the effectiveness of PrEP regimens.

Further, their status was evaluated at 14, 21, and 28 days after treatment, and at each point, the numbers of hospitalization were significantly lower in the treated group.

The investigators, from Washington University School of Medicine in St. Louis, note that the responses measured were one-third as strong as those mounted by healthy individuals.
The FDA has also converted the indication for pembrolizumab from an accelerated to a full, regular approval for patients with urothelial carcinoma.

FDA’s green light for biopharmaceutical company’s drug candidate builds upon previous approval to treat pancreatic cancer.
Because patients may interact with a pharmacist more regularly than other health care providers, it is possible to detect potential signs and symptoms of atrial fibrillation in a patient during their encounters.
Brigham and Women’s Hospital conducted an analysis to measure the response of T cells from MMR and Tdap vaccines that could help to decrease COVID-19 severity.
Experts from Emory Healthcare and Winship Cancer Institute discuss the management of oncology patients by pharmacists within an NCI-designated comprehensive cancer center.

In an interview with Pharmacy Times®, Guillermo Torre-Amione, MD, PhD, FACC, the chairman of Cardiol Therapeutics, discusses the potential use for CBD in hospitalized patients with COVID-19, as well as its therapeutic benefits in certain patients with cardiovascular disease.

Patricia C. Kienle, RPh, MPA, BCSCP, FASHP, director of Accreditation and Medication Safety at Cardinal Health and member of US Pharmacopeia’s (USP) Compounding Expert Committee, and Nicole Palmer, the senior manager of USP Volunteer Operations, discuss USP’s volunteer program.
The AHA worked with a team of leading health authorities to develop the advisory, which highlights recommendations, algorithms, and guidance for health care professionals and researchers who specialize in heart and brain health.
Investigators from Oregon Health and Science University (OHSU) said that it is crucial to emphasize the importance of vaccinations, as well as the maintenance of public health measures that cut off the spread of the virus.

Investigators found that 65% of patients with cancer and COVID-19 were hospitalized and 17% required admission or transfer to a higher level of care.