
Using chromosome genomic array testing in combination with existing risk stratification models can better determine patients with myelofibrosis who can benefit from transplant.

Luke Halpern is an assistant editor with Pharmacy Times. Luke wrote for Pharmacy Times in the summer of 2023, and assumed a full-time role in June 2024. His work has been featured in Pharmacy Times and the American Journal of Managed Care. He graduated from the University of Massachusetts, Amherst in May 2024.

Using chromosome genomic array testing in combination with existing risk stratification models can better determine patients with myelofibrosis who can benefit from transplant.

The new data demonstrates the effectiveness of Mim8 in children with hemophilia A regardless of inhibitor status, expanding the populations in which the novel drug in development could have a treatment benefit.

There was an association observed between fluctuating low-density lipoprotein cholesterol and the risk of developing dementia and cognitive impairment.

Semaglutide has been approved for reducing kidney disease progression and cardiovascular risk in patients with type 2 diabetes, marking a significant advancement in CKD management.
Priority review status will allow for expedited development of zongertinib, putting it on the path toward approval as the first in a new class of drugs for mutated NSCLC.
Identifying and managing IVIG-related side effects early on in treatment can improve efficacy and safety for patients with CIDP.
Prescribing and dosing appropriate treatments for patients with sickle cell disease while managing episodes of acute pain are some of the integral roles of pharmacists.

New data from the longest known trial of pediatrics and adolescents in the postacute phase of COVID-19 show they are at a heightened risk of gastrointestinal tract symptoms.

Donna Ryan explains why long-term treatment with GLP-1 medication is typically necessary to maintain weight loss benefits.
The approval in multiple indications is designed to improve access to effective, proven treatments for skeletal fractures, which can greatly reduce patient quality of life.

Steven Pipe, MD, explains the long-term efficacy data surrounding etranacogene dezaparvovec in patients with hemophilia B.

Amlenetug could provide a treatment option for patients with multiple system atrophy, a progressive and rare condition that causes damage to the brain’s nerve cells.
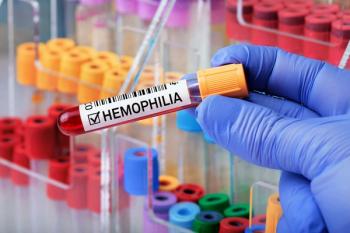
A one-time infusion of etranacogene dezaparvovec-drlb led to sustained bleeding control and a reduction in factor IX prophylaxis in patients with hemophilia B.

PP-01 could become a first-in-class treatment for patients with cannabis use disorder.

Therapies like the newly approved suzetrigine, a nonopioid NaV1.8 pain signal inhibitor, could revolutionize pain management and mitigate the risks associated with opioid use.
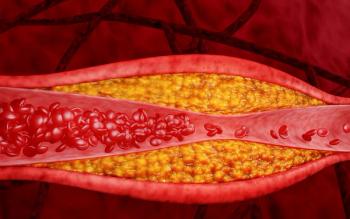
The agency will now review the novel PCSK9 inhibitor for possible approval in patients with or at high risk of atherosclerotic cardiovascular disease.
Building on a previously granted orphan drug designation, the latest FDA action for amezalpat puts the drug in a position for regulatory approval.

Cytopenic myelofibrosis is characterized by poorer prognosis and more severe anemia and thrombocytopenia compared with proliferative myelofibrosis.

The approval is based on positive clinical trial results that indicated deep and durable reductions in plexiform neurofibroma.
Emerging data indicate that COVID-19 is also characterized by inflammation present in the heart and other organs

Richard Lewis discusses the critical role pharmacists and health care providers play in ensuring safe administration of IVIG for patients with CIDP.
Thalassemia, a chronic blood condition that reduces hemoglobin levels, requires a hands-on approach from pharmacists in patient monitoring and diagnosis.

Circulating lactate is increased in patients with myelofibrosis and corresponds with the remodeling of lactate export channel monocarboxylate transporter 4, suggesting a link with fibrosis establishment.
BR55, an injection of perfluorobutane/nitrogen lipopeptide-coated microbubbles, could aid in the detection of angiogenesis and allow for earlier diagnosis in patients with Crohn disease.

Pharmacists should educate patients on abelacimab's potential therapeutic impact on atrial fibrillation patients at risk of stroke.

Proper nutrition and awareness of potential side effects from GLP-1 medications are essential for patients undergoing treatment.
Compared with other treatments for myasthenia gravis, nipocalimab demonstrated more significant improvements in disability scores, with tolerable safety.
The designation builds on previous regulatory action for ADI-001 and allows for expedited development of the treatment for systemic lupus erythematosus.
Donna Ryan discusses the landscape of GLP-1 medications and the lineup of indications that the drug class has received indications for in recent years.

More accurate prognostic risk assessment could aid pharmacists in the management and counseling of patients with primary myelofibrosis.