
Subcutaneous immunoglobulin (SCIg) is a hopeful alternative to intravenous immunoglobulin in patients with inflammatory myositis due to a lack of adverse effects and fewer financial constraints.

Luke Halpern is an assistant editor with Pharmacy Times. Luke wrote for Pharmacy Times in the summer of 2023, and assumed a full-time role in June 2024. His work has been featured in Pharmacy Times and the American Journal of Managed Care. He graduated from the University of Massachusetts, Amherst in May 2024.

Subcutaneous immunoglobulin (SCIg) is a hopeful alternative to intravenous immunoglobulin in patients with inflammatory myositis due to a lack of adverse effects and fewer financial constraints.
Saad Usmani, MD, MBA, FACP, FASCO, explores treatment duration, patient characteristics, and toxicity profiles in transplant-ineligible patients with newly diagnosed multiple myeloma.
Results from the NeoADAURA trial demonstrate the sustained efficacy of osimertinib in patients with epidermal growth factor receptor-mutated (EGFRm) non-small-cell lung cancer (NSCLC).
Amanda Gronniger, PharmD, CPh, BCCP, discusses practical strategies pharmacists can use to improve cholesterol management, from lifestyle counseling to emerging therapies.
The FDA has approved darolutamide for patients with metastatic castration-sensitive prostate cancer after positive results in the ARANOTE clinical trial.

Chip Hailey, a 74-year-old pharmacist with hemophilia B, shares how gene therapy has liberated him from decades of challenging treatment and restored his independence.

The CDC has updated its COVID-19 vaccine guidance for children and pregnant individuals, sparking a debate among health experts and concerns over insurance coverage.
Amid intensified scrutiny of COVID-19 vaccines at HHS, Moderna’s mRNA-1283 COVID-19 vaccine was granted FDA approval for patients 65 years and older and patients aged 12 to 64 years with at least 1 or more underlying risk factors for severe COVID-19.

Saad Usmani, MD, MBA, FACP, FASCO, discusses the subgroup analysis of transplant-ineligible patients with newly diagnosed multiple myeloma from the CEPHEUS trial.
IVIG treatment significantly boosts platelet counts in pregnant people with thrombocytopenia, revealing key predictors for effective response and optimizing clinical decisions.
Building on positive low-density lipoprotein cholesterol (LDL-C)-lowering seen in patients with atherosclerotic cardiovascular disease, inclisiran was found to induce meaningful and sustained LDL-C reductions following recent acute coronary syndrome.

The announcement is set to affect multiple grants awarded to Moderna, which is developing mRNA-1018, a candidate vaccine against avian influenza.
V114, a 15-valent pneumococcal conjugate vaccine (PCV), provided better protection against serotypes 22F and 33F compared with 13-valent PCV (PCV13).

Derek Webb, PharmD, a community pharmacist and Virginia Board of Pharmacy member, provides comprehensive guidance on managing seasonal allergies, distinguishing between symptoms, and exploring treatment options for patients.
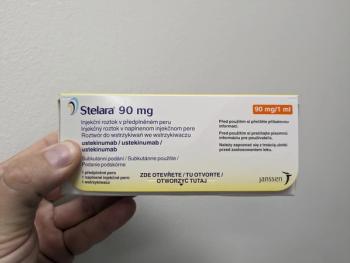
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.

The change is poised to impact countless individuals who may have sought a COVID-19 vaccine this upcoming respiratory season.
Following a meeting of the Vaccines and Related Biological Products Advisory Committee, the FDA announced its recommendation of COVID-19 vaccines targeting LP.8.1, a strain of the JN.1 variant that has become dominant in the US.

Pharmacists can make the process easier and more convenient for patients.
Interventions such as educational brochures, provider-delivered educational programs, and computerized reminders were found to help improve pneumococcal vaccine uptake in older adults.
With each increase in lipoprotein(a) level in patients with atherosclerotic cardiovascular disease (ASCVD), there was a corresponding increase in risk of experiencing an ASCVD event.
Additional intravenous immunoglobulin (IVIG) treatment shows promise for patients with Kawasaki disease who are resistant to infliximab, potentially matching the efficacy of second IVIG doses in patients resistant to IVIG.

In a major policy shift, officials from the FDA announced a new regulatory framework for COVID-19 vaccinations, prioritizing adults 65 years and older and individuals with serious comorbidities putting them at high risk for severe COVID-19.

A systematic review reveals declining pediatric pneumococcal complicated pneumonia rates following the introduction of pneumococcal conjugate vaccine 13.
The vaccine, indicated with a restriction on use for those younger than 65 years and without comorbidities, was proven safe and effective in a series of large clinical trials, and marks the first protein-based vaccine to be approved in the United States.
The regimen, which consists of durvalumab and Bacillus Calmette-Guerin, offers new hope to patients with high-risk non-muscle-invasive bladder cancer.

A new study reveals that heart failure patients with implantable devices are more likely to receive the pneumococcal conjugate vaccine 13, impacting vaccination rates and mortality.
In patients with synchronous metastatic colorectal cancer treated without curative intent, high low-density lipoprotein (LDL) cholesterol was associated with poor survival.

Logan Schneider, MD, highlights the importance of integrating patient-reported outcomes into clinical trials to better capture the full therapeutic impact of low-sodium oxybate (Xywav) beyond traditional sleep metrics.
The FDA approved belzutifan, the first oral treatment for pheochromocytoma and paraganglioma, offering a new treatment pathway for patients with these rare tumors.
New trial results reveal icotrokinra's effectiveness in achieving significant skin clearance for challenging scalp and genital psoriasis.