
Research highlights the impact of sleep disturbances and vasomotor symptoms (VMS) on HRQoL in women transitioning to menopausal period, emphasizing the need for effective management.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

Research highlights the impact of sleep disturbances and vasomotor symptoms (VMS) on HRQoL in women transitioning to menopausal period, emphasizing the need for effective management.

Embedding standards in pharmacy practice help ensure medication quality, safety, and patient understanding.
When treating vasomotor symptoms (VMS), 15- and 20-mg doses of estetrol (E4) did not significantly hinder postmenopausal women’s blood pressure, including those with cardiovascular risks.

New research reveals how menopausal hormone therapy impacts cognitive health in postmenopausal women, highlighting the importance of administration routes and estrogen types.

Elinzanetant was effective in women with low vasomotor symptom (VMS) burden and those with endocrine therapy-caused VMS.

Prior to the 2025 American Heart Association (AHA) Scientific Sessions Meeting, Craig J. Beavers, PharmD, FACC, FAHA, FCCP, BCCP, BCPS (AQ-Cardiology), CACP, discusses two recent papers that are influencing practice within the cardiovascular space.

Compared with late postmenopausal women, menopausal hormone therapy (MHT) may influence certain Alzheimer disease-related biomarkers in early postmenopausal women.

The mean time intervals between injection and complete retinal vascularization were similar between the biosimilar and its reference product.

Whether they are collaborating with other health care professionals, helping with medication adherence, or educating patients, pharmacists have a crucial role in every setting.
These negative effects were primarily presented when cells were stressed by inflammatory proteins in patients with chronic kidney disease (CKD) with APOL1 mutations.

Research reveals the impact of SSRIs on pregnancy, highlighting risks and benefits for maternal mental health and fetal development.
TACTI-004 follows 2 clinical trials, TACTI-002 and INSIGHT-003, which also assessed efti with pembrolizumab in first-line treatment of non–small cell lung cancer (NSCLC).

The approved indication now includes adults with moderately to severely active ulcerative colitis and Crohn disease who have not been treated with tumor necrosis factor blocking agents.
Higher levels of cyclic adenosine monophosphate (cAMP) may be key to diagnosing asthma and its severity as well as monitoring patients.

FDA approves Uzedy, an extended-release injectable formulation of risperidone, enhancing treatment options for adults with bipolar I disorder.

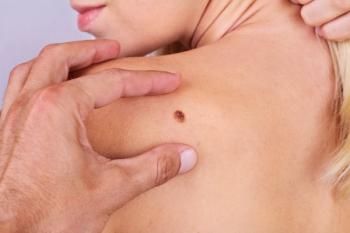
Cemiplimab's approval is supported by findings from the C-POST clinical trial.

This is the first pediatric FDA approval for golimumab, according to the manufacturer.
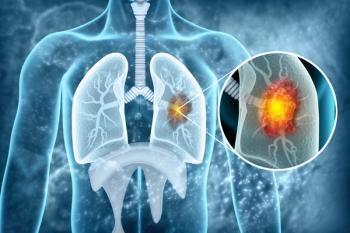
Aumolertinib and osimertinib show similar efficacy and safety when treating patients with EGFR-mutant non–small cell lung cancer (NSCLC), providing valuable insights for treatment optimization.

Recent research confirms that rheumatoid arthritis patients in remission can safely switch to the biosimilar infliximab-dyyb, offering a cost-effective treatment option.
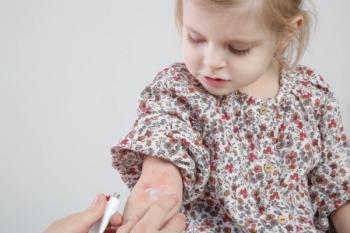
The approval is supported by findings from the INTEGUMENT-PED trial and the INTEGUMENT-OLE long-term extension study.
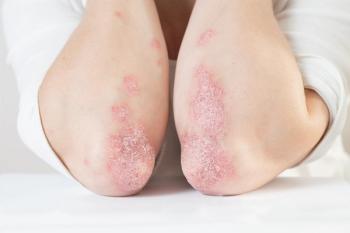
A phase 3 study shows SYSA1902, a biosimilar to Stelara, is clinically equivalent for treating moderate to severe plaque psoriasis.

This is the second generic version of mifepristone to be approved by the FDA.
Recent findings reveal that stereotactic radiation therapy (SABR) offers long-term survival outcomes comparable to surgery in patients with early non–small cell lung cancer (NSCLC).
In chronic kidney disease (CKD), SDMA showed a stronger connection to vascular health compared with ADMA.

In this video, experts who attended the 2025 International Myeloma Society (IMS) Annual Meeting highlighted topics of interest as well as pivotal data.

BATURA demonstrated that albuterol/budesonide significantly reduced severe exacerbation risk reduction in patients with mild asthma.
Patients with newly diagnosed transplant-eligible multiple myeloma (NDTE-MM) had high rates of complete response and minimal residual disease–negativity.
Interim trial data presented at the 2025 World Conference on Lung Cancer shows pumitamig’s promising efficacy in patients with extensive stage small cell lung cancer (ES-SCLC).
The approval is supported by clinical findings from the EMBER-3 trial.