Particularly, younger women with chronic kidney disease (CKD) experienced the greatest survival disadvantage.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

Particularly, younger women with chronic kidney disease (CKD) experienced the greatest survival disadvantage.

Emergency medicine pharmacists discuss how they used a gamified, PowerPoint-based virtual escape room to improve disaster preparedness training, strengthen critical thinking, and highlight the essential role of pharmacists in emergency management.

The findings from the BATURA clinical trial were presented at the 2025 ACAAI Annual Scientific Meeting.

The biosimilars can be used to treat osteoporosis and cancer-related bone loss in certain populations.

The research further emphasizes the need for more attention on both chronic kidney disease (CKD) and mental health conditions.

The approval is based on results from the phase 3 DeLLphi-304 clinical trial.

Depemokimab shows significant improvements in asthma symptoms and peak expiratory flow, supporting its twice-yearly use for severe asthma management.

With this action, Thrombate III has become the first and only antithrombin concentrate approved for adult and pediatric patients with hereditary antithrombin deficiency (hATd).

The Annual Meeting Highlighted Emerging Research on Hormone Therapy, Brain and Heart Health, Disparities in Menopause Care, and the Expanding Role of Pharmacists
Patients with non–small cell lung cancer (NSCLC) had an objective response rate of about 77%.
Home monitoring enhances pediatric asthma management, boosting patient engagement and awareness while providing valuable medical feedback for better control.
New findings reveal lorundrostat significantly lowers blood pressure and albuminuria in patients with chronic kidney disease, enhancing standard treatment efficacy.

The FDA approves Poherdy, a new biosimilar to Perjeta, enhancing treatment options for HER2+ breast cancer patients with affordability and efficacy.

Although further data will be presented in 2026, lifileucel shows promise as a 1-time treatment for advanced non–small cell lung cancer (NSCLC).

Razumab, a ranibizumab biosimilar, shows comparable efficacy to its innovator counterpart in treating myopic choroidal neovascular membrane (mCNVM), offering a cost-effective solution.

Pharmacists enhance community health by providing accessible care, managing complex medications, and fostering trust in patient relationships.
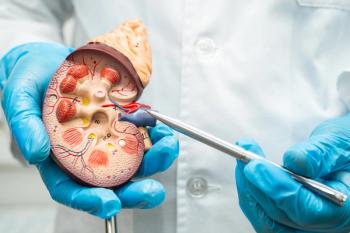
Research highlights significant barriers to contraceptive use and reproductive health management for women with chronic kidney disease (CKD), urging improved nephrology care.

These findings are from the WAYFINDER clinical trial and were presented at the CHEST 2025 Annual Meeting.

Although the incidence of vascular assess-associated infections (VAIs) was low, the incidence caused by noncuffed catheters (NCC) was high.

Two experts discuss sessions that covered perimenopause, premature ovarian insufficiency, GLP-1–based treatments, and the health needs of Indigenous and Hispanic women.

The FDA designates Stoboclo and Osenvelt as interchangeable biosimilars for denosumab, enhancing treatment options for osteoporosis and cancer-related conditions.

FDA designates Conexxence and Bomyntra as interchangeable denosumab biosimilars, enhancing access and affordability for patients needing critical therapies.
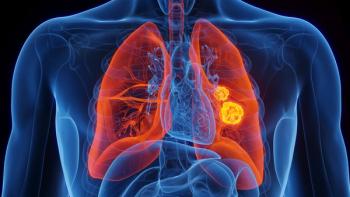
Data from multiple KEYNOTE clinical trials support the sustained survival benefits of pembrolizumab in non–small cell lung cancer (NSCLC).

Christina Barrington, PharmD, champions pharmacists' vital role in enhancing health care access and quality, advocating for their recognition as essential providers.

A new low-carbon albuterol inhaler shows therapeutic equivalence to original options, suggesting reduced emissions and improved sustainability for patients with asthma and other respiratory conditions.
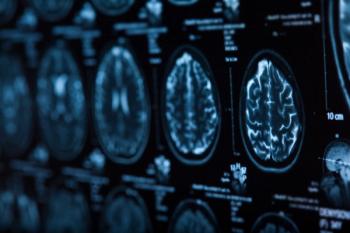
Research highlights the link between earlier menopause, cardiac function, and brain health, emphasizing the need for sex-specific dementia risk strategies.

Research highlights the impact of provider type on menopause treatment, revealing a need for standardized education to improve care quality for women.
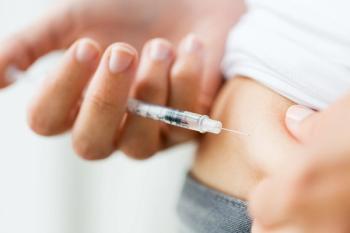
The authors wrote that their findings show a necessary update following early data from the Women’s Health Initiative.

Further research is needed to better understand which characteristics may better predict who are more likely to benefit from estrogen-based menopausal hormonal therapy (MHT) for anxiety.
New clinical trial results suggest G-CSF as a promising alternative for managing hot flashes in menopausal women, without the risks of traditional therapies.