
In addition to increased prevalence, the age of diagnosis has also decreased.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

In addition to increased prevalence, the age of diagnosis has also decreased.

Study results suggest young participants who survived cancer have difficulty in areas such as memory, attention, and ability to process information compared with those who did not have cancer.

The FDA cited human factors unrelated to the drug's efficacy as the reason for rejection.

The authors suggested that future research investigate emerging blood-based biomarkers and explore temporal associations between sleep duration and Alzheimer disease biomarker changes.

The FDA prioritizes centanafadine's review for ADHD treatment, offering a novel, once-daily oral option for diverse patient needs.

CMS announces the selection of 15 high-cost drugs for Medicare price negotiations, aiming to reduce prescription costs for older adults and taxpayers.

Recent research reveals no link between menopause hormone therapy and dementia risk, urging further studies to clarify its effects on cognitive health.

The study found that anxiety and depression in those with Parkinson disease were significantly associated with fatigue severity.
Recent research links depression to increased risk of Lewy body dementia and Parkinson disease, emphasizing the need for early detection and awareness.

A study determined that lifestyle factors and comorbidities were more likely to be associated with the onset of diabetes.

Pharmacists enhance health care delivery by managing medication refills, addressing drug shortages, and supporting chronic disease management.

Recent research reveals no significant link between prenatal acetaminophen use and increased risks of autism or ADHD in children, challenging FDA claims.

Preeclampsia significantly raises the risk of chronic kidney disease (CKD) later in life, highlighting the need for targeted postpartum monitoring for affected women.
According to the news release, the manufacturers have 30 days to respond.

AbbVie partners with the Trump administration to enhance drug affordability and access, committing to significant investments in US pharmaceutical innovation.

The treatment was granted a Prescription Drug User Fee Act target action date of May 10, 2026.

The findings reemphasize a need for mental health support for those in the pharmacy profession.
Recently released guidelines enhance chronic kidney disease management, emphasizing early detection and innovative treatments for better patient outcomes.
The pivotal clinical trial supports the concomitant administration of tirzepatide and ixekizumab, which significantly improves outcomes for patients with psoriatic arthritis (PsA) and obesity.
Study authors describe a framework to help patients with chronic kidney disease (CKD) change and adhere to diets.
Surveys reveal significant misunderstandings among Americans about shared decision-making in childhood vaccinations, highlighting the need for better public education.
The guidelines are believed to promote screening adherence by providing women with a more comfortable, accessible, and less stressful testing option.

Federal vaccine recommendations for pediatric patients shift dramatically, reducing routine immunizations from 17 to 11, sparking debate on safety and efficacy.

The FDA approval of Boncresa and Oziltus, new denosumab biosimilars, enhances access to treatments for osteoporosis and cancer-related bone loss.
New research highlights the efficacy and safety of HRS-1780, an investigational nonsteroidal MRA, in treating chronic kidney disease (CKD).

Research reveals that wildfire smoke exposure significantly impacts pediatric asthma control in the northeastern US, highlighting urgent climate health concerns.

Narsoplimab gains FDA approval for treating transplant-associated thrombotic microangiopathy, showing promising survival rates in high-risk patients.

The FDA's 2025 biosimilar approvals enhanced treatment options for chronic diseases, improving accessibility and affordability for patients and health care providers.
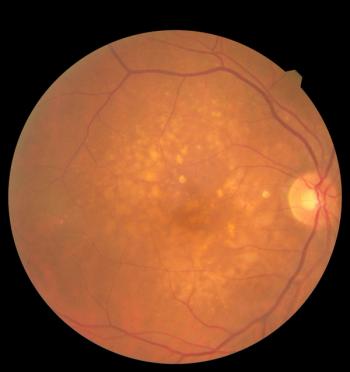
Nufymco, the FDA-approved ranibizumab biosimilar, enhances treatment options for retinal diseases, improving patient access and affordability.
A high-fiber, plant-based diet shows promise in delaying multiple myeloma progression and enhancing metabolism and immune response in patients with precursor conditions.
Published: July 15th 2025 | Updated:
Published: July 8th 2025 | Updated:
Published: December 23rd 2025 | Updated:
Published: June 6th 2025 | Updated:

Published: September 11th 2025 | Updated:
Published: March 5th 2024 | Updated: