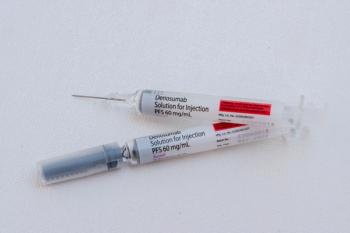
When treating patients with bone metastases, the biosimilar demonstrated equivalent safety and efficacy to its reference product.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

When treating patients with bone metastases, the biosimilar demonstrated equivalent safety and efficacy to its reference product.

A new biosimilar, LY05008, shows comparable efficacy and safety to dulaglutide in managing type 2 diabetes mellitus (T2DM) in Chinese adults.
New research confirms that switching from tocilizumab to its biosimilar, Avtozma, maintains efficacy and safety in patients with rheumatoid arthritis (RA).
Both preclinical and translational evidence support MET inhibition as a therapeutic approach in patients with small cell lung cancer (SCLC).

The complete response letter (CRL) stated that vatiquinone's new drug application (NDA) did not provide substantial evidence of efficacy and would need to be evaluated in a new trial.
Unicycive Therapeutics secures a US patent for UNI-494, a promising treatment for chronic kidney disease, enhancing its development and partnership potential.

FDA approves Tonmya, the first new fibromyalgia treatment in 15 years, offering hope for effective pain relief and improved quality of life.
The designation is supported by promising results from the ongoing phase 1/2 BEACON clinical trial.
New research links high perilipin 2 expression in lung adenocarcinoma to aggressive disease progression and shorter survival, highlighting potential treatment targets.

New research reveals that novel biomarkers enhance risk prediction for kidney failure and mortality in chronic kidney disease (CKD), paving the way for personalized treatment.

Pharmacists help connect patients with the correct resources and help remove barriers so patients can receive continuous care.

Pharmacists can educate patients and health care professionals in addition to coordinating lab orders, interpreting results, and guiding patients through the next steps.

The action follows 2 FDA-issued complete response letters.

Jennifer Goldman, PharmD, CDCES, BC-ADM, FCCP, explains the different stages of type 1 diabetes, teplizumab’s use, and emphasizes the pharmacist’s role in care.
Discover effective strategies for managing obesity to enhance cardiovascular health, including innovative treatments and the vital role of pharmacists.

The researchers speculate that this is because of the antioxidant and anti-inflammatory properties of coffee and tea.

Recent data link a higher consumption of ultraprocessed food to increased lung cancer risk, highlighting the need for dietary changes and further research.

The FDA approved fremanezumab for pediatric migraine prevention, offering a new treatment option for children and adolescents aged 6 to 17 years.

Herzuma shows promising efficacy and safety as a cost-effective biosimilar for treating HER2+ advanced gastric cancer, offering new hope for patients.

The FDA approves aceclidine ophthalmic solution 1.44%, the first aceclidine eye drop for presbyopia, offering a new solution for millions struggling with near vision loss.
The authors note that the findings can have significant public health implications given the widespread water chlorination and increased chronic kidney disease burden.
A recent study reveals Mvasi's comparable safety profile to Avastin for retinal diseases, highlighting the need for on-label biosimilars in ophthalmology.

The treatment proved efficacy across all 3 key markers of C3 glomerulopathy (C3G) and primary immune complex membranoproliferative glomerulonephritis (IC-MPGN).
A phase 2 trial shows oxylanthanum carbonate (OLC) effectively manages hyperphosphatemia in patients with chronic kidney disease (CKD), offering a promising treatment option.
The combination regimen had stronger benefits in patients with locally advanced or metastatic epidermal growth receptor-mutated (EGFRm) non–small cell lung cancer (NSCLC) than osimertinib alone.

GLP-1 receptor agonists show promise in alleviating PCOS symptoms, including weight loss and menstrual regulation, despite needing further research.
There were also no observed differences in retention rates or safety among patients with ankylosing spondylitis.

New research reveals uranium isotopes in urine as potential biomarkers for kidney damage, highlighting risks from contaminated drinking water in the US.
Results from the ZENITH trial show sotatercept's promise for pulmonary arterial hypertension treatment.

The complete response letter (CRL) did not raise safety concerns, but stated the IGNYTE trial was inadequate and did not provide substantial evidence of effectiveness.