
Meloxicam injection gains FDA approval for effective management of moderate-to-severe pain, offering a nonopioid alternative in pain control strategies.

Meloxicam injection gains FDA approval for effective management of moderate-to-severe pain, offering a nonopioid alternative in pain control strategies.

The FDA approves clesrovimab-cfor, a new RSV vaccine, offering infants effective protection against severe respiratory illness during their first RSV season.
The FDA has approved a groundbreaking single-pill combination therapy for hypertension, enhancing cardiovascular health with triple-agent efficacy.
The FDA has approved darolutamide for patients with metastatic castration-sensitive prostate cancer after positive results in the ARANOTE clinical trial.

Clairity Breast's FDA-approved AI platform revolutionizes breast cancer risk prediction, enhancing early detection and personalized prevention strategies.
Amid intensified scrutiny of COVID-19 vaccines at HHS, Moderna’s mRNA-1283 COVID-19 vaccine was granted FDA approval for patients 65 years and older and patients aged 12 to 64 years with at least 1 or more underlying risk factors for severe COVID-19.
The FDA designated Hadlima as an interchangeable biosimilar to Humira, enhancing patient access and potential savings for various conditions.

The FDA grants fast track designation to TEV-53408, a promising treatment for celiac disease designed to address gluten intolerance and improve patient outcomes.

The formulation allows for individualized treatment in children with adrenocortical insufficiency.

FDA approves acoltremon ophthalmic solution, a groundbreaking treatment for dry eye disease, enhancing natural tear production and patient quality of life.
The FDA approved the MenQuadfi vaccine for young children, enhancing protection against invasive meningococcal disease and its severe complications.
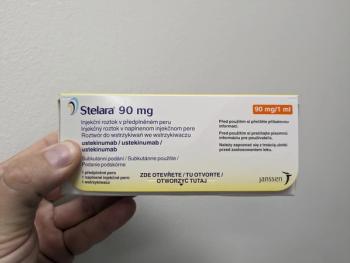
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.

The change is poised to impact countless individuals who may have sought a COVID-19 vaccine this upcoming respiratory season.
Following a meeting of the Vaccines and Related Biological Products Advisory Committee, the FDA announced its recommendation of COVID-19 vaccines targeting LP.8.1, a strain of the JN.1 variant that has become dominant in the US.

The approval is based on findings from the INSPIRE trial, where treprostinil demonstrated efficacy in pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD).

This indication is for adult and pediatric patients aged 12 and older with plaque psoriasis of the scalp and body.
Mepolizumab gains FDA approval as a promising add-on therapy for COPD patients with eosinophilic phenotype, reducing exacerbation rates significantly.
Ranibizumab injection (Susvimo; Genentech) could offer long-lasting vision preservation and reduce the need for frequent injections in patients with diabetic retinopathy.

Yuflyma gains FDA interchangeability with Humira, enhancing patient access to effective biosimilar treatments for various inflammatory conditions.

In a major policy shift, officials from the FDA announced a new regulatory framework for COVID-19 vaccinations, prioritizing adults 65 years and older and individuals with serious comorbidities putting them at high risk for severe COVID-19.
The approval expands the therapy’s indication to children as young as 7 years.
The Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio is the first in vitro diagnostic device that tests blood to aid in diagnosing Alzheimer disease.
The vaccine, indicated with a restriction on use for those younger than 65 years and without comorbidities, was proven safe and effective in a series of large clinical trials, and marks the first protein-based vaccine to be approved in the United States.

The FDA approves retifanlimab for advanced anal cancer, offering new hope with improved survival rates and treatment options for patients.
Cure Rare Disease's CRD-002 receives FDA orphan drug designation, advancing hope for SCA3 treatment with innovative oligonucleotide therapy.

The approved injection contains dihydroergotamine (DHE), which is the same medication used in hospitals, in a ready-to-use device.
Emrelis is a groundbreaking treatment for advanced non-small cell lung cancer, targeting high c-Met protein overexpression.
The FDA approved belzutifan, the first oral treatment for pheochromocytoma and paraganglioma, offering a new treatment pathway for patients with these rare tumors.

The new at-home test aims to increase comfort and screening timeliness through a more accessible method.

With the FDA approval, avutometinib/defactinib is now the first and only FDA-approved treatment for patients with recurrent low-grade serous ovarian cancer (LGSOC).