A phase 1/2 clinical trial will provide crucial data on the vaccine's tolerability and pave the way for future development and potential widespread use.

A phase 1/2 clinical trial will provide crucial data on the vaccine's tolerability and pave the way for future development and potential widespread use.
The antibody-drug conjugate is currently undergoing evaluation in a phase 1 trial that enrolled patients with pancreatic cancer and squamous non-small cell lung cancer (NSCLC).
Currently, BEAM-302 is undergoing a phase 1/2 clinical trial to evaluate its efficacy in patients with alpha-1 antitrypsin deficiency (AATD) who have lung disease and liver disease.
Approval of durvalumab is in combination with gemcitabine and cisplatin as neoadjuvant treatment for adults with muscle invasive bladder cancer.

Approval of fitusiran, a small interfering RNA drug, was found effective in patients with hemophilia regardless of inhibitor status.

The biosimilar is approved for all indications of the reference products, Prolia and Xgeva.

The approval fills a treatment gap for patients with this rare disease, which can severely diminish quality of life.
Patients achieved statistically significant benefits in progression-free survival and overall response rates.

Gepotidacin is indicated for female patients who are at least 12 years of age and weigh at least 40 kg with uncomplicated urinary tract infections (uUTIs) caused by certain microorganisms.
If approved, this novel monoclonal antibody therapy could become a standard of care emergency option for patients with acute myocardial infarction, which affects millions in the United States and across the world.

Sjögren's disease is one of the most common chronic autoimmune diseases that affects 4 million individuals around the world.

The new indication for guselkumab in Crohn disease builds off a previous approval in ulcerative colitis, providing patients a treatment option for the major forms of inflammatory bowel disease.
Vutrisiran becomes the first and only therapeutic FDA-approved to treat cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults.


The agent is the only approved therapy designed to address the underlying mechanisms of the disease.

The combination was approved for treatment of patients with gastroesophageal junction (GEJ) adenocarcinoma.

Exidavnemab is a novel monoclonal antibody with disease-modifying potential.

Patients with a disorder from this family of neurodevelopmental conditions face a lack of approved treatment options, making radiprodil poised to transform the treatment paradigm.
The treatment is indicated for patients aged 6 and older with hypertension, the reduction of stroke in hypertension and left ventricular hypertrophy, and diabetic nephropathy in certain patients with type 2 diabetes.

The new indication would include adults with heart failure with a left ventricular ejection fraction (LVEF) of 40% or higher, such as mildly reduced or preserved LVEF.
Eculizumab, a humanized monoclonal antibody, was first approved for adults with generalized myasthenia gravis in 2017.

Currently, EN001 is undergoing evaluation in a phase 1b trial.

The treatment is currently undergoing evaluation in an investigational preclinical program and is projected to be in human trials in 2026.

The treatment's expanded indication for chronic kidney disease (CKD) is expected to be available by April 2025.
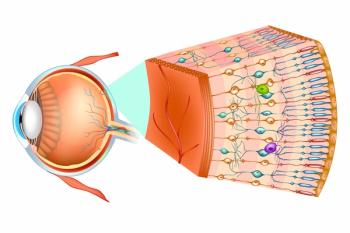
With the new designation, OpCT-001 can receive expedited investigations into its potential to improve vision function in patients with primary photoreceptor diseases.
The approval is based on results from 2 phase 3 clinical trials that showed revakinagene taroretcel’s efficacy in macular telangiectasia type 2 (MacTel).

The move is expected to expand clozapine access for patients with schizophrenia.

According to a news release, this marks the first significant innovation in epinephrine delivery for this patient population is more than 35 years.

The designation was granted after positive results from the phase 1 STOMP-I clinical trial (NCT01915927).
The FDA is issuing new changes to labels on testosterone products, clarifying that there are no increases in major cardiac events among men with hypogonadism but noting an increase in blood pressure with the use of such products.