Building on a previously granted orphan drug designation, the latest FDA action for amezalpat puts the drug in a position for regulatory approval.

Building on a previously granted orphan drug designation, the latest FDA action for amezalpat puts the drug in a position for regulatory approval.

Avacincaptad pegol intravitreal solution is a prescription eye injection used to treat geographic atrophy.

Risdiplam is the only approved non-invasive disease-modifying SMA treatment.

The approval follows the ECHELON-3 study, which enrolled adults with relapsed or refractory large B-cell lymphoma.

The approval is based on positive clinical trial results that indicated deep and durable reductions in plexiform neurofibroma.
Rezpegaldesleukin is designed to balance the body’s immune system response through activation of regulatory T-cells.
If approved, brensocatib will be the first and only approved treatment for bronchiectasis and the first within a new class of medicines, called dipeptidyl peptidase 1 inhibitors.
BR55, an injection of perfluorobutane/nitrogen lipopeptide-coated microbubbles, could aid in the detection of angiogenesis and allow for earlier diagnosis in patients with Crohn disease.
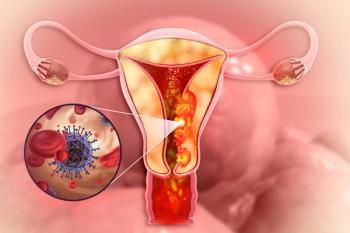
The indication is for patients with endometrial cancer who may benefit from treatment with ACR-368 (Acrivon Therapeutics).
The decision offers patients with antimicrobial resistance, who have limited or no treatment options, a new alternative.
The designation builds on previous regulatory action for ADI-001 and allows for expedited development of the treatment for systemic lupus erythematosus.
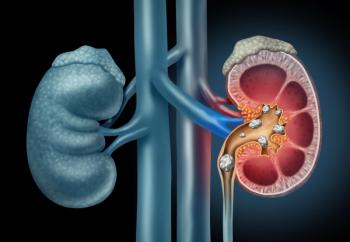
ABO-101 is supported by preclinical data that demonstrated significant reductions in urinary oxalate in PH1 disease models.

Diabetic macular edema (DME) is the leading cause of new blindness in the US.

The approval marks the first subcutaneous infusion device approved for the treatment of Parkinson disease, providing a new option for patients with serious disease who are unresponsive to other therapies.

Tocilizumab-anoh is expected to increase competition, improve patient access, and deliver cost savings in the treatment of various inflammatory conditions.

The regenerative medicine advanced therapy designation expedites the development and review of regenerative medications that have the potential to address unmet needs for serious or life-threatening diseases.

The approval includes indications for rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and COVID-19.

Meloxicam and rizatriptan reduces migraine pain and help patients return to normal functioning with efficacy through 24 to 48 hours after a single dose for some patients.

Apitegromab is a muscle-targeted therapy for improvement of motor function in individuals with spinal muscular atrophy.

The supplemental new drug application is based on objective response rate and duration of response results from a phase 2 study that assessed the efficacy and safety of belzutifan.

This marks the first US biologic license application filing acceptance for a biosimilar candidate to golimumab.

This follows the approval of everolimus tablets for patients aged 1 and older in January 2025.

MB-105 is a first-in-class CD5-targeted chimeric antigen receptor (CAR) T-cell therapy for the treatment of relapsed or refractory CD5-positive T-cell lymphoma.
Preliminary phase 1 trial results demonstrate that patients with small cell lung cancer generated early clinical activity after being administered ZL-1310.

The new indication for semaglutide adds to the long list of conditions that the GLP-1 receptor agonist has been approved for, and provides a new option for high-risk patients with these chronic conditions.
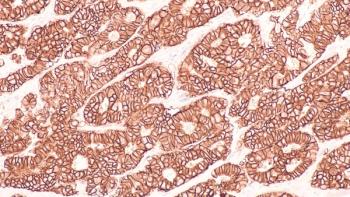
The antibody-drug conjugate shows efficacy in reducing disease progression risk and increasing progression-free survival in patients with HR+, HER2-low, or HER2-ultralow metastatic breast cancer.
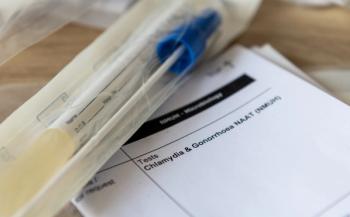
The cobas liat system is a closed system that aims to reduce contamination risks and enhances the reliability of results at the point of care.
Administered every 4 weeks, the approval could serve as a breakthrough for easier and more effective care for patients with early Alzheimer disease.

Based on the updated label, new pharmacodynamic data have been added for a better understanding of the drug for individuals with attention-deficit/hyperactivity disorder (ADHD).

The fast-track designation follows the FDA orphan drug designation that was granted for DYNE-101 in September 2023 for the same treatment population.