
Combination therapy with both nivolumab and relatlimab leads to increased T-cell activation compared with monotherapy with either agent alone.

Combination therapy with both nivolumab and relatlimab leads to increased T-cell activation compared with monotherapy with either agent alone.
Analysis included a 29% objective response rate in cohort 4 of the C-144-01 study, the company says.
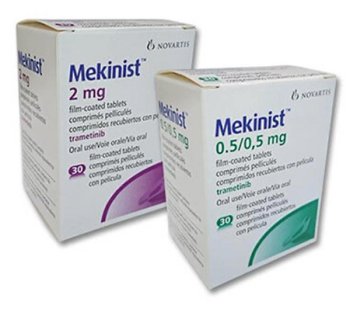
Trametinib (Mekinist) is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA-approved test.
Because of the likelihood that patients with melanoma will develop adverse effects on therapy, it is crucial for pharmacists to proactively educate patients and provide them with tools to help mitigate and manage these issues.
Vusolimogene oderparepvec is in development for the treatment of multiple skin cancers either alone or in combination with anti-PD1 therapy.
The FDA previously granted Fast Track Designation for the combination of lifileucel and pembrolizumab in metastatic melanoma.
Cutaneous squamous cell carcinoma should be diagnosed early and treated promptly to avoid complications.

Relatlimab plus nivolumab (Opdualag) shows improved progression-free survival compared with nivolumab monotherapy in patients with unresectable or metastatic melanoma.
Kimmtrak found to be the first therapy to demonstrate a survival benefit for HLA-A*02:01–positive adult patients with unresectable or metastatic uveal melanoma.

Early-stage melanomas at risk of spreading secrete the TGFβ2 growth factor, which causes the downregulation of the AMBRA1 and Loricrin proteins.

The FDA granted priority review to the combination of relatlimab and nivolumab in September 2021 based on the results of this study.
Study results establish LAG-3 inhibition as therapeutically relevant third immune checkpoint pathway

Dupilumab has been a breakthrough treatment for patients with atopic dermatitis, but future studies are needed to continue learning about its strength.

Melanoma is responsible for 80% of skin cancer deaths.

Commonly used medications may influence responses to checkpoint inhibitors among patients with cancer.

FoundationOne CDx is a next-generation sequencing based in vitro diagnostic device for detection, insertion and deletion alterations, and copy number alterations in 324 genes and select gene rearrangements.

Pembrolizumab is the first anti-PD-1/anti-PD-L1 therapy to demonstrate a recurrence-free survival benefit in the adjuvant setting for melanoma.
Approximately 40% of patients with stage IV melanoma have brain metastases at diagnosis.
Shannon M. Rotolo, PharmD, BCPS, examines the role dupilimab plays as a disease-modifying therapy for the treatment of moderate-to-severe atopic dermatitis, and Peter Lio, MD, considers the unmet needs that exist within atopic dermatitis therapy.
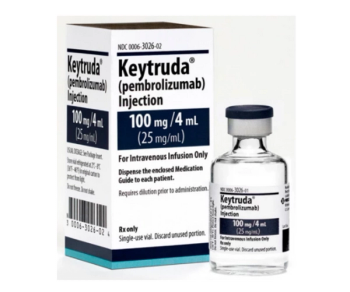
Pembrolizumab (Keytruda) can be used for multiple cancer types, including melanoma, non-small cell lung cancer, and head and neck squamous cell cancer.

The investigators said that these cells, referred to as lymph node resident memory T cells, have been demonstrated to counteract the spread of melanoma in mice.
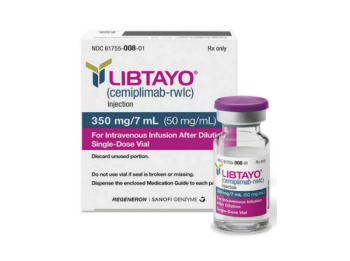
Cemiplimab is a recombinant human immunoglobulin G4 monoclonal antibody that binds to PD-1 and blocks its interaction with PD-L1 and PD-L2.

Relatlimab is the first LAG-3-blocking antibody to demonstrate a clinical benefit for patients with melanoma in phase 3 data.

Phase 3 trial results show that the drug reduced the risk of death or reoccurrence by 35% for individuals with high-risk disease compared with the placebo.

Survey participants saw potential for immunotherapy use in earlier stages of several diseases, including melanoma, lung cancer, and bladder or urothelial cancer.