There are gonadotoxic risks of chemotherapy, radiation, and surgery that are important to discuss with patients.


There are gonadotoxic risks of chemotherapy, radiation, and surgery that are important to discuss with patients.
The implementation of accountable care organizations (ACOs) also worsened disparities in any emergency department or hospital use for Medicaid-insured children with asthma.

Research funding that aims to understand impacts of systemic racism on pediatric asthma should include plans to undo policies that maintain inequities created by systemic racism.
This review discusses the significance of the FDA-approved drug inotuzumab ozogamicin in pediatric patients with acute lymphocytic leukemia (ALL) and relapsed/refractory (R/R) ALL, and the evolving therapeutic options in R/R ALL.

Patients aged 7 years and older can now be treated for their cataplexy or excessive daytime sleepiness with the extended-release oral formulation.
The increase was observed across multiple subgroups, including those with overweight or obesity and hospitalized patients.

Pharmacists also help patients manage their adverse effects and treat patients to live healthy lives.

Oral immunotherapy is a growing area of research that involves ingesting increasing doses of the allergen to desensitize the patient’s immune system.

Although the findings indicate a potential for predicting asthma or wheezing onset, these trends cannot yet be used for early diagnoses in individual children.

Video chatting, texting, watching videos, and playing video games were activities most commonly associated with depressive symptoms in adolescents.

Significant polarization surrounding COVID-19 vaccinations in late 2020 and early 2021 looked to have had an impact in increasing vaccine hesitancy.
Research showed that children born via assisted reproductive technology were 36% more likely to develop heart defects.
By utilizing a lower dose, patients can avoid potential adverse events and high financial burden.

Pharmacists and health care providers can counsel around vitamin D supplements and natural intake to increase a child’s vitamin D consumption.
The findings support current recommendations for pregnant individuals or those who might become pregnant during the influenza season, specifically those with successive pregnancies.
Treatment for primary coenzyme Q10 (CoQ10) deficiency can include high-dose oral CoQ10 supplementation, but not all patients respond to this treatment.
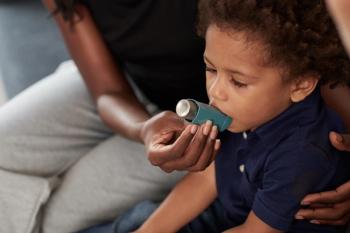
A recent study found that indoor allergen exposure, including mouse and cockroach allergens, led to an increased risk of respiratory viral infections in children with asthma.

MDL-101 is a proposed novel precision medicine that targets the LAMA1 gene, causing LAMA2 congenital muscular dystrophy type 1a.
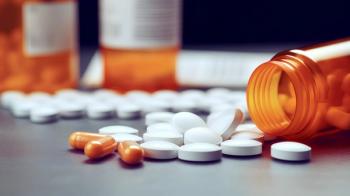
The guidelines mark a shift in practice by recommending a multimodal approach to opioid prescribing practices.

Investigators found a significant association for febrile seizures, but not with epilepsy.

Treatment is typically tailored to the individuals, with HCT being the only curative treatment bone marrow failure, but long-term outcomes are generally poorer due to toxicities.
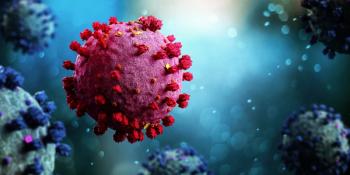
Investigators reported that more than half of individuals had great knowledge about protecting themselves from COVID-19.
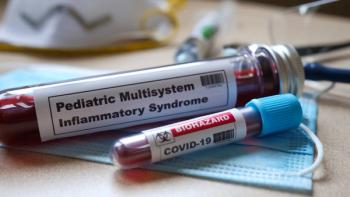
Further trials are required to examine the long-term feasibility of intravenous immunoglobulin (IVIG) for multisystem inflammatory syndrome.
Implementation of yearly mock code training can reinforce essential knowledge and increase pharmacists’ clinical competence and confidence when faced with actual neonatal and pediatric emergencies.

The authors urge that screening, prevention, and treatment of anxiety and depression should be important health care priorities for youth who have chronic pain.