
The announcement came hours after the companies disclosed that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) had issued a positive opinion to authorize the vaccine.

The announcement came hours after the companies disclosed that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) had issued a positive opinion to authorize the vaccine.
Pharmacy Times® interviewed Michael Flannery, PharmD, the assistant director of pharmacy operations at the University of Rochester Medical Center, on the concerns he has regarding the COVID-19 vaccination process ahead.
The action allows for medicinal products to be authorized on a conditional basis for seriously debilitating or life-threatening diseases or for use in emergency situations in response to public health threats in the European Union.
Based on the pickup rates in the study, the investigators said kiosks could provide pharmacies significant annual savings because patients picked up an additional 400 prescriptions per year.
The ACIP advises the CDC on the populations and circumstances for which vaccines should be used.
Moderna’s COVID-19 vaccine (mRNA-1273) has been granted Emergency Use Authorization (EUA) by the FDA, making it the second vaccine to be issued an EUA for combating the virus.

Pharmacy Times® interviewed Sue Peschin of the COVID-19 Vaccine Education and Equity Project on the role of pharmacies in vaccinating the public with the COVID-19 vaccines.

Pharmacy Times® interviewed Sue Peschin, president and CEO of Alliance for Aging Research, on the work that the COVID-19 Vaccine Education and Equity Project is doing to engage diverse constituencies on the importance of the clinical trials process, regulatory review, and equitable access to COVID-19 vaccines.
Pharmacy Times® interviewed Sue Peschin, president and CEO of Alliance for Aging Research, on the work that the COVID-19 Vaccine Education and Equity Project is doing to support education about and equitable access to COVID-19 vaccines.

The additional doses bring the EC’s confirmed order commitment to 160 million doses, which will commence from the company’s dedicated European supply chain.

The COVID-19 pandemic has accelerated both acceptance and payment of novel care delivery models including telehealth.

Pharmacists can now play a larger role in increasing vaccination rates, while pharmacies can continue to be safe and convenient settings in which patients can receive vaccines.
If granted an EUA by the FDA, mRNA-1273 would become the second COVID-19 vaccine to receive the designation.
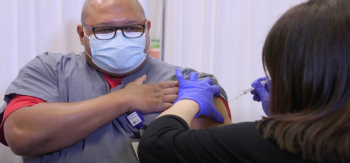
Pharmacists, physicians, and nurses have been among the first to receive COVID-19 vaccinations at health care facilities across the country.

Pfizer’s COVID-19 vaccine vials are supposed to hold 5 doses, but using a sixth or seventh dose could expand the supply by up to 40%.

Pharmacy Times® interviewed John Beckner, the senior director of strategic initiatives at the National Community Pharmacists Association, on how pharmacists can help people come back for the second dose of the COVID-19 vaccine.

Pharmacy Times® interviewed John Beckner, the senior director of strategic initiatives at the National Community Pharmacists Association, on some of the challenges community pharmacies may need to manage when receiving and administering COVID-19 vaccines.

Pharmacy Times® interviewed John Beckner, the senior director of strategic initiatives at the National Community Pharmacists Association, on the work needed to prepare for the arrival and administration of COVID-19 vaccines.

Results of Ellume’s rapid at-home COVID-19 test are sent to a smartphone in 15 minutes or less.

There are many benefits to adopting and integrating new technological methods for hosting pharmacy organization events.

Tips and tricks to make mask-wearing less problematic during the COVID-19 pandemic.

Although cytokine storms are usually associated with severe COVID-19, study indicates less severe disease may also be at risk of cytokine storm.

A thorough understanding of immunization billing processes for Medicare, Medicaid, and other third-party payers will be critical to ensure pharmacy staff can efficiently serve their communities and facilitate a smooth reimbursement process.
Among a subset of participants in the COVID-19 vaccine clinical trials, 0.63% of individuals in the vaccinated group had hypersensitivity-related adverse events that may represent allergic reactions.
Under the 340B Prime Vendor Program, the Prime Vendor has several functions that it carries out to support stakeholders in operating and navigate the field so that they can effectively achieve lower 340B drug pricing for those who need it most.