
Ranibizumab-eqrn is the only biosimilar approved for all 5 Lucentis indications.


Ranibizumab-eqrn is the only biosimilar approved for all 5 Lucentis indications.

FDA accepts supplemental Biologics License Application for Hyrimoz HCF, a biosimilar with the same indications as adalimumab (Humira).

From a patient perspective, the lack of awareness of biosimilars is the biggest barrier to adoption.

Indications for adalimumab include ankylosing spondylitis, Crohn disease, chronic plaque psoriasis, juvenile idiopathic arthritis, moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

With the rapid pace of change in biosimilar payer benefit design, efforts to stay regularly updated on policy changes ahead of time become critical for patient care.

Suzanne Soliman, PharmD, BCMAS welcomes Sonia T. Oskouei, PharmD, BCMAS, DPLA to discuss biosimilars within the pharmacy industry.

The treatment gives patients with neovascular age-related macular degeneration a more affordable option, the companies say.

Biosimilar therapeutic interchange processes can mitigate high costs of cancer drugs.
Many health care systems are turning to biosimilars to provide clinical outcomes that are similar to those provided by reference products, but at a reduced cost.

Pegfilgrastim-pbbk is the third oncology biosimilar developed by Amneal to gain FDA approval this year.
Health systems must have a flexible workflow in place to allow the use of non-preferred agents to accommodate payer demands and reduce financial toxicity.

Sonia T. Oskouei, PharmD, vice president of biosimilars at Cardinal Health, discussed how pharmacists can impact biosimilar adoption.
US segment to hit $121.8 billion in 2022, notes market research firm.
Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, discusses what biosimilars in the pipeline are expected to launch this year.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.
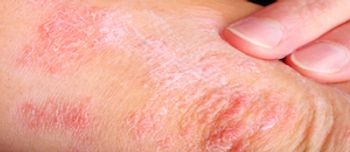
The phase 3, multicenter, randomized, double-blinded, comparative clinical study was evaluating the efficacy and safety of ABP 654 compared with ustekinumab in adult patients with moderate to severe plaque psoriasis.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.

Bevacizumab-maly (Almysys; mAbxience) is the third biosimilar of bevacizumab (Avastin) approved in the United States.

David Pope, PharmD, CDE, chief innovation officer at OmniSYS, discusses some of the technology trends that occurred in the pharmacy space during the pandemic.

Biosimilars could save consumers more than $100 billion in the coming years.

With the FDA approval and launch of the first interchangeable biosimilar in the United States in 2021, retail pharmacists are poised to play an increasing role in the future uptake of biosimilars.

In an interview with Pharmacy Times®, Chelsee Jensen, PharmD, pharmaceutical formulary manager at Mayo Clinic, discusses biosimilar usage in health systems and challenges that come with it.

In an interview with Pharmacy Times®, Eric Tichy, PharmD, MBA, BCPS, FAST, FCCP, Vice Chair of Pharmacy/Formulary at Mayo Clinic, discusses some formulary trends in biosimilars at the Academy of Managed Care Pharmacy Annual Meeting.

Shannon Hough, director of ClinReview and Clinical Content for McKesson, discusses the advantages of including pharmacy staff as part of the care team when payer or drug availability challenges arise.

Shannon Hough, director of ClinReview and Clinical Content for McKesson, discusses the advantages of including pharmacy staff as part of the care team when payer or drug availability challenges arise.